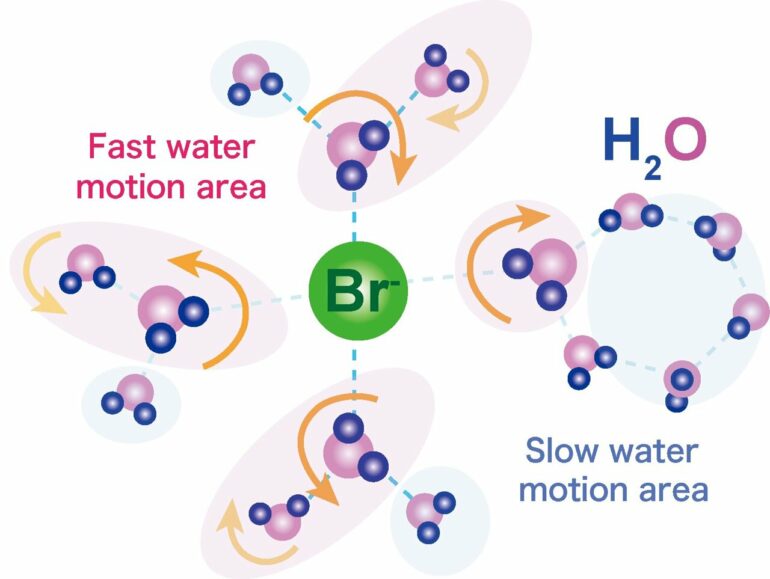
The way that water molecules behave in proton conducting materials is very important for understanding—and making the most of—their properties. This means being able to look at very rapid snapshots to catch changes in the water motion.
Researchers from Osaka University have taken a close look at semiclathrate hydrate crystals using quasi-elastic neutron scattering (QENS). The article, “Quasi-elastic neutron scattering studies on fast dynamics of water molecules in tetra-n-butylammonium bromide semiclathrate hydrate,” was published in Applied Physics Letters.
Semiclathrate hydrates have water molecule frameworks that house other molecules or ions as “guests” in their structures. The overall properties of the framework can therefore be controlled and tailored to particular requirements by introducing different guests.
However, some of the best proton conductors are highly acidic solutions and are difficult to be handled. Solid electrolyte alternatives are therefore needed. Tetra-n-butylammonium bromide (TBAB) semiclathrate hydrate is known to be a promising solid electrolyte, but the mechanism behind its performance has been unclear.
The researchers took a close look at the water molecule dynamics in TBAB semiclathrate hydrate using QENS. This allowed motions of the water molecules to be captured over much shorter periods than have been achieved with other techniques, providing a clearer picture of what is happening.
“The transfer of protons in the semiclathrate hydrate is suspended by the water molecules,” explains study lead author Jin Shimada. “The way the water molecules then reorient—their reorientation motion—then tells us about what might be affecting the conduction.”
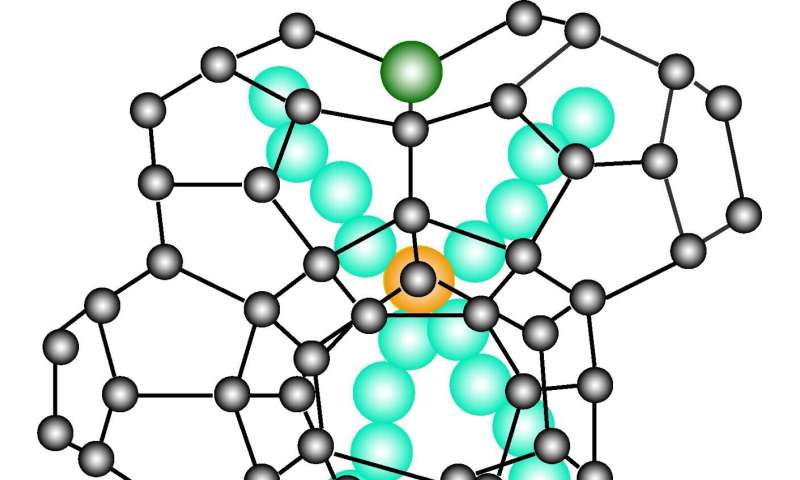
Schematic illustration of the crystal lattice of a semiclathrate hydrate. Black balls represent oxygen atoms in the water molecules, which are connected through hydrogen bonds (black lines). Inside the cage are the cations and anions (bromide ions) and the cage structure is formed by water molecules. © Takeshi Sugahara
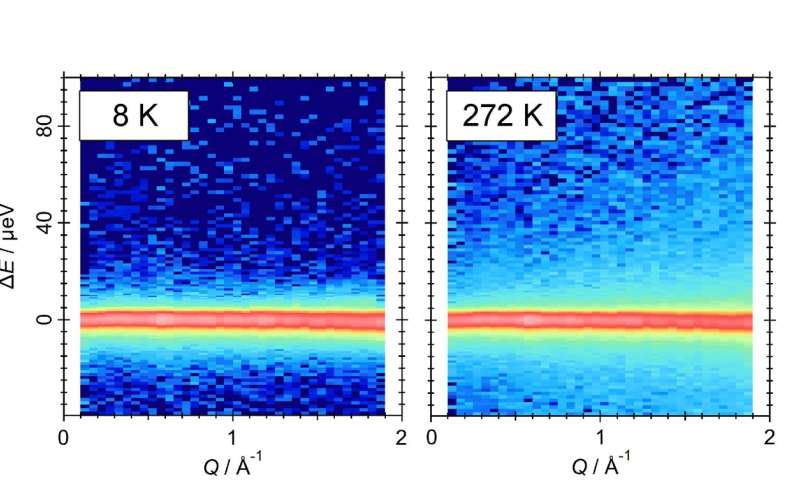
Changes in quasi-elastic neutron scattering of water molecules in TBAB semiclathrate hydrate. The left and right figures show the measurement results at cryogenic temperatures and at 272 K, respectively. The relatively bright light-blue region reflects the motion of water molecules. © 2023, Shimada et al., Quasi-elastic neutron scattering studies on fast dynamics of water molecules in tetra-n-butylammonium bromide semiclathrate hydrate, Applied Physics Letters
QENS showed that water molecules in the crystal reorientate themselves very rapidly in much shorter times than have previously been measured. In addition, the energy needed to prompt the change is consistent with that needed to break a hydrogen bond, the type of interaction that occurs between the guest ions and the water molecules.
It is believed that the large bromide ion that forms part of TBAB activates the water to behave as it would around bromide in aqueous solution.
“The insight we have gained into TBAB semiclathrate hydrate provides an excellent grounding for future innovation,” says senior author Takeshi Sugahara. “We believe the findings will contribute to the development of batteries and thermal storage materials.”
More information:
Jin Shimada et al, Quasi-elastic neutron scattering studies on fast dynamics of water molecules in tetra-n-butylammonium bromide semiclathrate hydrate, Applied Physics Letters (2023). DOI: 10.1063/5.0157560
Citation:
Bromide ions cause ripples in semiclathrate hydrates, finds neutron scattering study (2023, July 26)