New research from the UK National Nuclear Laboratory and the University of Liverpool in the UK has used a novel technique to explore how thermal oxidation affects the structure of a key component of nuclear reactors so that they can be engineered to operate for longer than before. This research was reported in Nuclear Engineering and Design.
The key component is graphite. It’s made up of tiny crystals that together form “grains” or “filler particles”. The grain size, along with the porosity, are used to characterize nuclear graphite into different grades. It plays an important role in many types of nuclear reactor. It is used in the current operating fleet of reactors that have supplied around 20% of the UK’s carbon-neutral electricity since the 1950s and could soon be used in advanced reactors which are planned to play a vital role in future environmentally friendly energy systems around the world.
Researchers at the UK National Nuclear Laboratory and the University of Liverpool examined the porosity of superfine grained nuclear graphite and linked this to a chemical change known as thermal oxidation. They focused on the very high temperatures some advanced reactors, known as high temperature reactors (HTR), will produce. The findings can be used to design efficient nuclear reactors that can last for much longer than the original fleet.
Nassia Tzelepi, Senior Fellow at the National Nuclear Laboratory, explains the significance of this work, “Thermal oxidation is one of the two mechanisms in HTR graphite components that can affect the performance of the reactors. Good understanding of its evolution and the effect on the graphite properties is required to qualify a graphite for use in an HTR.”
Inside a nuclear reactor
A nuclear reactor relies on neutrons to split atoms, releasing large amounts of energy. The neutrons move very quickly so a moderator is used to slow them down, making them more likely to be absorbed by an atom in the fuel. Solid blocks of graphite are excellent moderators.
A coolant extracts the energy and sends it somewhere it can be used. Existing reactors send the coolant to a turbine to produce electricity; future reactors might be used to generate low-carbon heat required by some industries. In a high temperature gas reactor (HTGR) helium gas is used as a coolant, reaching temperatures of more than 700 °C. Helium, even once it’s been purified, contains substances such as air or moisture. These impurities, combined with the high temperatures, can gradually cause the graphite to oxidize over the predicted 40 to 60-year life of a reactor.
Oxidation can change the porosity of the graphite, a key characteristic that affects its performance. Somewhat paradoxically, the extent of oxidation depends on the porosity: how the pores are linked together, their size, and the overall permeability of the material. Oxidation can be caused by high temperatures, known as thermal oxidation, or by radiation.
British scientists have a wealth of experience of oxidation caused by radiation in the historic fleet of graphite moderated reactors which operate at around 350 to 450 °C. Research now focuses on what would happen at the higher temperatures that a new generation of reactors would produce.
Examining the microstructure using a specialist technique
Understanding what happens to graphite isn’t straightforward. Like many other composite materials, both the manufacturing process and the type of raw materials play a role in the properties of the final product.
Graphite is made up of tiny crystals that typically have random orientations. Different grades of graphite can be produced that are characterized by the average size of the filler particle and the porosity. According to Nassia, “Every graphite is different depending on the raw materials and how it’s manufactured. We wanted to know what makes grades of graphite with seemingly the same attributes behave differently under thermal oxidation.” There are many possible reasons for the different behavior, and these can be investigated by looking at the microstructure under a microscope.
Nassia’s team focused their study on superfine grained graphite with an average grain size of 10–20 micrometers, because these are being considered for the next generation reactors. Using a high-temperature furnace and a carefully controlled atmosphere, four grades of superfine grained graphite were oxidized at 700–800 °C.
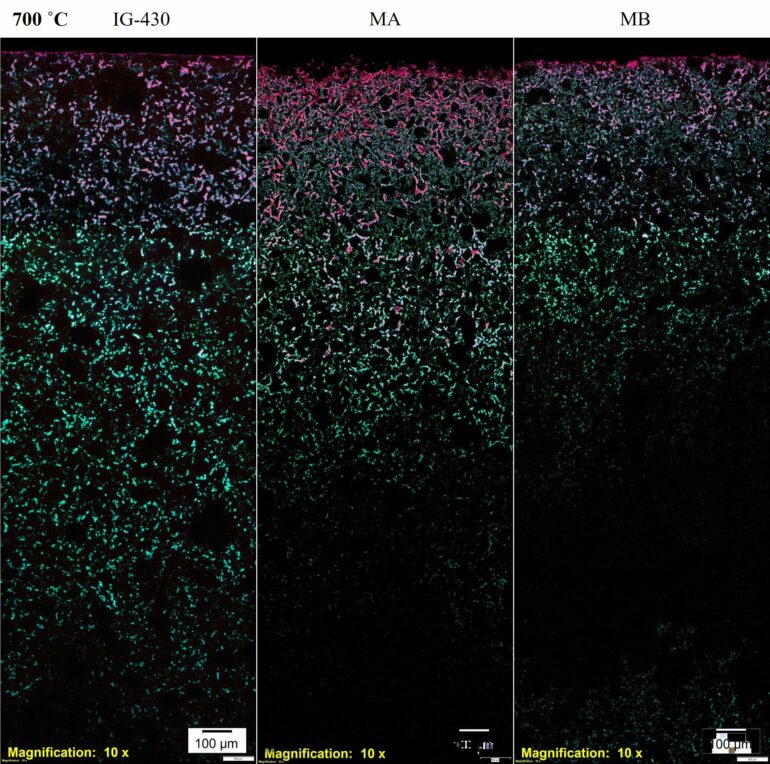
A technique developed specifically by British scientists to analyze nuclear graphite from the historic reactors was adapted to penetrate the smaller pores that exist in superfine grained graphite. This technique involves saturating “open” pores with a fluorescent dye. These open pores can be penetrated by the reactor coolant so it’s important to distinguish them from “closed” porosity that is not accessible. Using a microscope, images of the open and closed pores were collected and analyzed.
By combining this technique with other standard techniques clues were found to explain the different behavior. The overall depth of oxidation, shown in the graphic for three separate samples, was linked to the presence of relatively large, interconnected pores but at the surface of the graphite block a fine network of narrow, open pores enhanced the oxidation near the surface.
The team also found that the oxidation rate appeared to be lower in graphite where some parts of the microstructure had clumped together to form small agglomerates. When the grains clump together in this way there are fewer exposed edged that could be oxidized.
All this information about the microstructure can be used to determine exactly how graphite will perform inside future reactors. For Nassia, this new research shows why continued learning and discovery—and the creation of novel techniques—is important: “The UK has been operating graphite moderated reactors that are subjected to high levels of ‘radiolytic’ oxidation for over 50 years. Although the mechanism of radiolytic oxidation is different, a lot of experience can be drawn on to understand not only the effect of oxidation on the graphite properties but also to develop the methods to study it.”
The insight gained from these novel techniques supports the development of future reactors, ensuring that they consistently operate in optimal condition and allowing them to play a leading role in a future that relies completely on carbon-neutral energy.
More information:
Athanasia Tzelepi et al, Evolution of the microstructure of superfine grain graphites under thermal oxidation, Nuclear Engineering and Design (2023). DOI: 10.1016/j.nucengdes.2023.112421
Provided by
National Nuclear Laboratory
Citation:
New insights into high temperature oxidation of superfine nuclear graphite for future clean energy (2023, July 19)