According to the International Energy Agency (IEA), global hydrogen demand is expected to reach 530 million tons in 2050, a nearly six-fold increase from 2020.
Currently, the primary method of hydrogen production involves the reaction of natural gas and water vapor, resulting in what is known as gray hydrogen due to its carbon dioxide emissions, constituting around 80% of total hydrogen production. In contrast, green hydrogen is produced through water electrolysis using electricity, without emitting carbon dioxide. However, a challenge lies in the inevitable use of expensive precious metal catalysts, such as iridium oxide.
A research team led by Dr. Yoo Sung Jong of the Hydrogen and Fuel Cell Research Center at the Korea Institute of Science and Technology (KIST) have succeeded in significantly reducing the cost of green hydrogen production by implementing an anion exchange membrane water electrolysis device with excellent performance and durability by introducing a carbon support.
Carbon supports have been utilized as supports for various electrocatalysts due to their high electrical conductivity and specific surface area, but their usage has been limited because they readily oxidize to carbon dioxide in water electrolysis conditions, specifically at high voltages and in the presence of water.
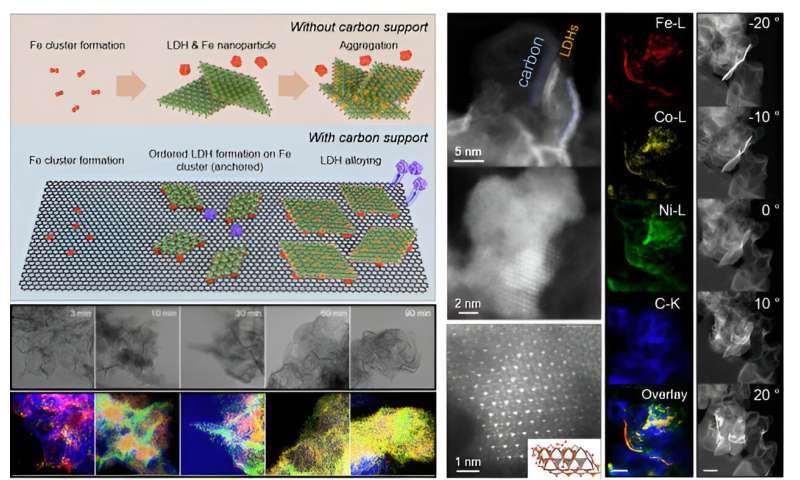
Time-dependent-lapse transmission electron micrograph images of nickel-iron-cobalt layered double hydroxide synthesis on carbon support, high resolution scanning TEM and EDS elemental mapping images. © Korea Institute of Science and Technology (KIST)
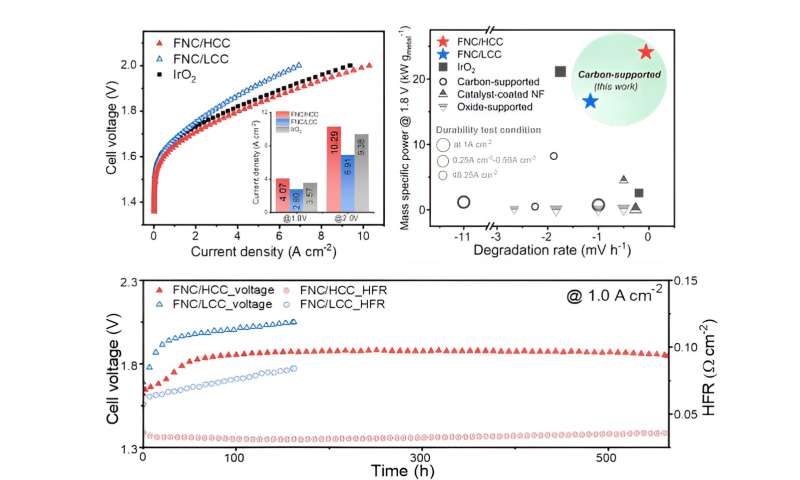
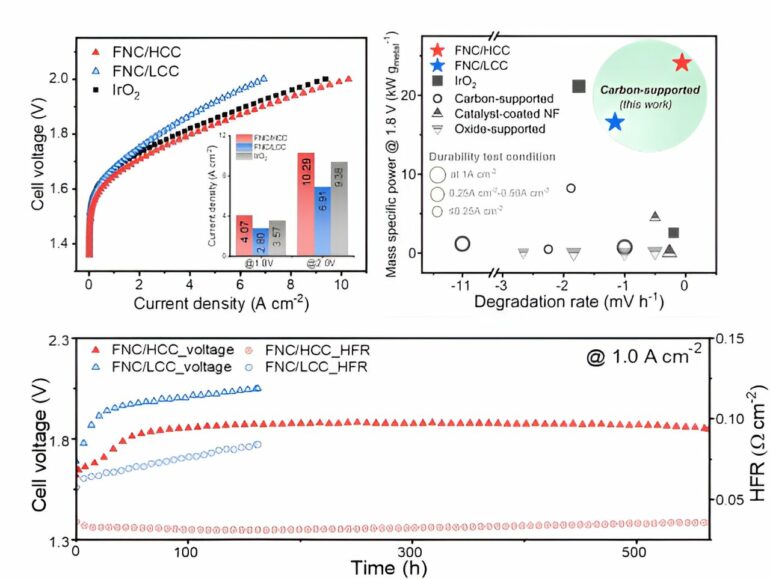
Electrochemical activity evaluation of nickel-iron-cobalt layered double hydroxide and single cell test results. © Korea Institute of Science and Technology (KIST)
The team synthesized a nickel-iron-cobalt layered double hydroxide material, a significantly cheaper alternative to iridium, on a hydrophobic carbon support and used it as an electrocatalyst for the oxygen evolution reaction in anion exchange membrane electrolysis. The catalyst showed excellent durability due to the layered structure facing a hydrophobic carbon support and a nickel-iron-cobalt layered double hydroxide catalyst.
In terms of carbon corrosion, it was found that the generation of carbon dioxide during the corrosion process was reduced by more than half, primarily because of decreased interaction with water, a key factor in carbon corrosion.
As a result of performance evaluation, it was found that the newly-developed catalyst support achieved a current density of 10.29 A/cm2 in the 2 V region, exceeding the 9.38 A/cm2current density of commercial iridium oxide, and demonstrated long-term durability of about 550 hours. The researchers also confirmed a correlation between electrolysis performance and the hydrophobicity of carbon, showing for the first time that the support’s hydrophobicity can significantly affect the water electrolysis device’s performance.
“The results of this research confirm the applicability of water electrolysis devices on carbon supports, which have previously been limited in use due to corrosion problems, and it is expected that water electrolysis technology can grow to the next level if the research focused on catalyst development is expanded to various supports.”
“We will strive to develop various eco-friendly energy technologies, including green hydrogen production,” said Dr. Yoo Sung Jong Yoo in KIST.
More information:
Myeong-Geun Kim et al, Realizing the potential of hydrophobic crystalline carbon as a support for oxygen evolution electrocatalysts, Energy & Environmental Science (2023). DOI: 10.1039/D3EE00987D
Provided by
Korea Institute of Science and Technology
Citation:
Study findings may dramatically lower the cost of producing green hydrogen (2023, October 4)