Aging is a biological process that no one can avoid. Ideally, growing old should be a time to relax and enjoy the fruits of your labor. Aging also has a darker side, however, often linked to disease.
Every second, your cells perform billions of biochemical reactions that fuel essential functions for life, forming a highly interconnected metabolic network. This network enables cells to grow, proliferate and repair themselves, and its disruption can drive the aging process.
But does aging cause metabolic decline, or does metabolic disruption accelerate aging? Or both?
To address this chicken-or-egg question, you first need to understand how metabolic processes break down during aging and disease. I am a scientist and researcher, and my lab focuses on exploring the complex relationship between metabolism, stress and aging. Ultimately, we hope this work will provide strategies to promote healthier aging and more vibrant lives.
Link between metabolism and aging
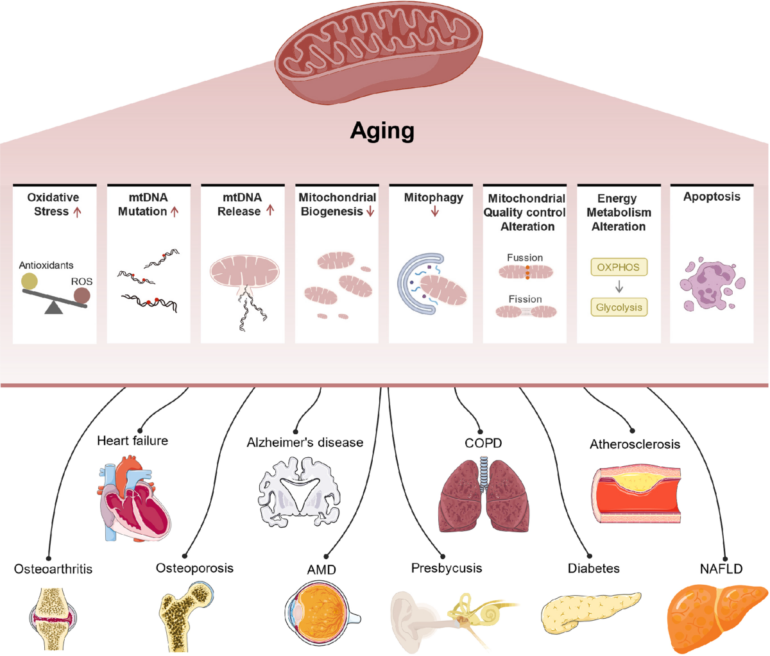
Aging is the most significant risk factor for many of society’s most common diseases, including diabetes, cancer, cardiovascular disease and neurodegenerative disorders. A key factor behind the onset of these health issues is the disruption of cellular and metabolic homeostasis, or balance. Disrupting homeostasis destabilizes the body’s internal environment, leading to imbalances that can trigger a cascade of health issues, including metabolic disorders, chronic diseases and impaired cellular functions that contribute to aging and other serious conditions.
Disrupted metabolism is linked to many hallmarks of aging cells, such as telomere shortening, which is damage to the protective ends of chromosomes, and genomic instability, the tendency to form genetic mutations.
Metabolism can be divided into two broad processes: anabolism, or building up molecules, and catabolism, or breaking down molecules.
A dysfunctional metabolism is also linked to poorly functioning mitochondria; cellular senescence, or when cells stop dividing; imbalances in gut microbes; and cells’ reduced ability to detect and respond to different nutrients.
Neurological disorders, such as Alzheimer’s disease, are prime examples of age-related conditions with a strong link between dysregulated metabolism and functional decline. For example, my research team previously discovered that in aging mice, the ability of bone marrow cells to produce, store and use energy is suppressed due to increased activity from a protein that modulates inflammation. This energy-deficient state leads to an increase in inflammation that’s worsened by these aging cells’ reliance on glucose as their main fuel source.
Experimentally inhibiting this protein in the bone marrow cells of aging mice, however, revitalizes the cells’ ability to produce energy, reduces inflammation and improves plasticity of an area of the brain involved in memory. This finding…