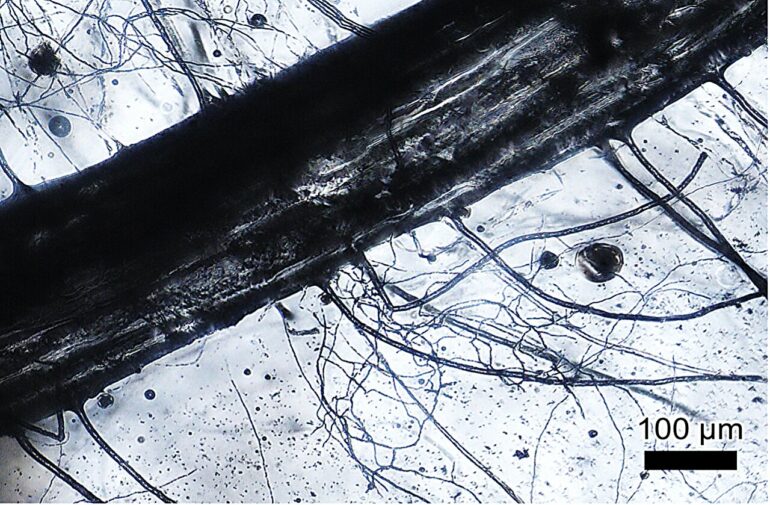
Microfluidic technology has become increasingly important in many scientific fields, such as regenerative medicine, microelectronics, and environmental science. However, conventional microfabrication techniques face limitations in scale and in the construction of complex networks. These hurdles are compounded when it comes to building more intricate 3D microfluidic networks.
Now, researchers from Kyushu University have developed a new and convenient technique for building such complex 3D microfluidic networks. Their tool? Plants and fungi.
The team developed a “soil” medium using nanoparticles of glass (silica) and a cellulose-based binding agent, then allowed plants and fungi to grow roots into it. After the plants were removed, the glass was left with a complex 3D microfluidic network of micrometer-sized hollow holes where the roots once were.
The new method can also be utilized for observing and preserving 3D biological structures that are typically difficult to study in soil, opening new opportunities for research in plant and fungal biology. Their findings were published in the journal Scientific Reports.
“The primary motivation for this research was to overcome the limitations of conventional microfabrication techniques in creating complex 3D microfluidic structures. The focus of our lab is biomimetics, where we try to solve engineering problems by looking to nature and artificially replicating such structures,” explains Professor Fujio Tsumori of Kyushu University’s Faculty of Engineering, who led the study.
“And what better example of microfluidics in nature than plant roots and fungal hyphae? So, we set out to develop a method that could harness the natural growth patterns of these organisms and create optimized microfluidic networks.”
The researchers began by developing a “soil” like mix for plants to grow in, but instead of dirt, they combined growth medium with glass nanoparticles smaller than 1 μm in diameter with hydroxypropyl methyl cellulose as a binding agent. They then seeded this “soil” mixture and waited for the plants to take root. After confirming successful plant growth, the “soil” was baked, leaving only the glass with root cavities.
“The process is called sintering, which aggregates fine particles together into a more solid state. It is similar to powder metallurgy in the manufacturing of ceramics,” continues Tsumori. “In this case it is the plant that does the molding.”
Their method was able to replicate the intricate biological structures of a plant’s main roots which can be up to 150 μm in diameter, and all the way down to it root hairs which can be about 8 μm in diameter. Tests with other organisms showed that the method can even replicate the root structure of fungi, called hyphae.
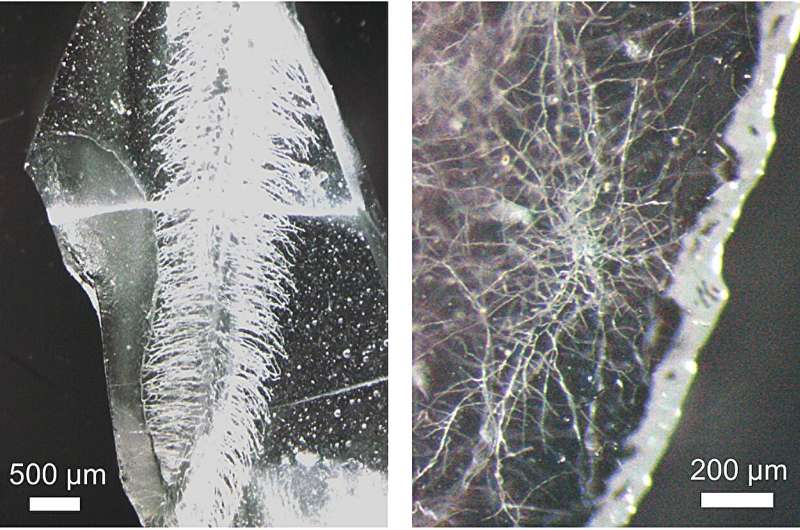
Glass structure of vasculature built from rye plant (left), and koji Aspergillus oryzae (right). Plants and hyphae-like cavities are formed inside the transparent glass chip. The root hair-like channels (left) are about 10 μm thick, and the hypha channels (right) are about 2 μm thick. © Kyushu University/Tsumori Lab
“Hyphae are even thinner and can be as small as 1-2 μm in diameter. That’s thinner than a single strand of spider silk,” says Tsumori.
The team hopes that their new bio-inspired microfluidic fabrication technique could be used in various fields of science and engineering, potentially leading to more efficient microreactors, advanced heat exchangers, and innovative tissue engineering scaffolds.
“In the biological sciences, this technique provides a unique tool for studying the intricate 3D structures of plant roots and fungal networks, which can advance our understanding of soil ecosystems,” concludes Tsumori.
“By bridging biological systems and engineering, our research has the potential to pave the way for new technologies and scientific discoveries.”
More information:
Tetsuro Koga et al, Replicating biological 3D root and hyphal networks in transparent glass chips, Scientific Reports (2024). DOI: 10.1038/s41598-024-72333-y
Citation:
Building roots in glass, a bio-inspired approach to creating 3D microvascular networks using plants and fungi (2024, November 19)