In a recent article published in Molecular Cell, Ali Shilatifard, Ph.D., the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics, and Yuki Aoi Ph.D., a postdoctoral fellow in Shilatifard laboratory, provided a comprehensive overview of the elongation stage of DNA transcription, its central role in the regulation of gene expression and how its dysregulation is associated with developmental defects, disease and aging.
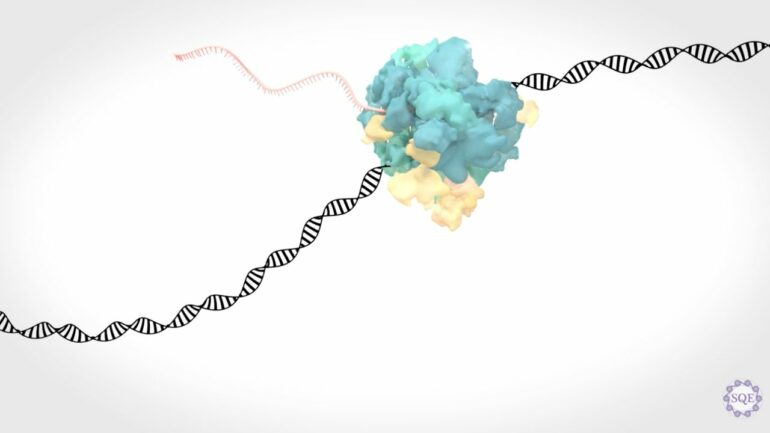
Transcription elongation, the process of synthesizing RNA from DNA, occurs when RNA polymerase II, a multiprotein complex, travels down a strand of DNA and copies genetic information from the DNA to RNA that is then converted into different proteins which fuel different cellular processes.
The regulation of transcription elongation heavily relies on a variety of intracellular factors seamlessly working together, as research from the Shilatifard laboratory and others have shown over the last two decades.
The connection between transcription elongation control and cancer was first discovered by Shilatifard in 1996 in a study published in Science, which demonstrated for the first time that transcription elongation control is a key regulatory step in the proper regulation of gene expression. This fundamental finding laid the groundwork for subsequent research which has helped illuminate how its disruption causes different developmental defects, diseases and even contributes to the aging process.
“What we showed almost 30 years ago is that elongation is a central regulatory step and that its misregulation can cause leukemia, and now we and others have shown how many different cellular processes use transcription elongation control as a key regulatory step,” Shilatifard said.
In the review, Shilatifard and Aoi provide a comprehensive outline of the current understanding of the mechanisms promoting transcriptional elongation control and highlight novel genetic engineering techniques that have helped them and other investigators uncover its unique biological properties.
The authors also discussed how recent in vivo studies, including those conducted in the Shilatifard laboratory, have contributed to an improved understanding of the intracellular factors that control transcription elongation, factors which may also serve as therapeutic targets for treating various diseases and that when targeted, could potentially inhibit or reverse accelerated aging.
“We and others have come across a series of fascinating discoveries that highlight the crucial role for transcriptional elongation by RNA polymerase II, also known as Pol II elongation, in gene regulation, as well as disease and aging,” said Yuki Aoi, Ph.D., a postdoctoral fellow in the Shilatifard laboratory and lead author of the editorial.
“We are confident that our article will assist in advancing future research that investigates both the mechanisms and the physiological relevance of Pol II elongation, ultimately leading to a better understanding of disease and aging processes,” Aoi said.
More information:
Yuki Aoi et al, Transcriptional elongation control in developmental gene expression, aging, and disease, Molecular Cell (2023). DOI: 10.1016/j.molcel.2023.10.004
Provided by
Northwestern University
Citation:
Exploring transcription elongation control in development, disease and aging (2023, November 3)