The sea is the world’s largest ecosystem, and it harbors two photosynthetic organisms that produce approximately half of the oxygen on Earth. The cyanobacterium Prochlorococcus is the most abundant photosynthetic organism in the oceans and fixes approximately 4 gigatons of carbon each year, comparable to the net global primary production of the world’s agriculture industry.
Photosynthesis relies on iron, the supply of which is limited in the ocean, and the remarkable ecological success of Prochlorococcus is based on its ability to thrive in low-nutrient waters.
Work led by Ivo Tews at the University of Southampton studied the Prochlorococcus iron-binding protein FutA using a number of complementary structural biology techniques, including serial crystallography at I24 and at SACLA.
The work demonstrates that FutA can accommodate iron in two different oxidation states, a functionality that is believed to make Prochlorococcus more efficient. The study is published in the Proceedings of the National Academy of Sciences.
As part of this work, X-rays, neutrons and visible light were all used to help understand iron binding in FutA. Neutron crystallography was used to locate hydrogen atoms around the iron binding site, which allowed determination of the charges of amino acid side chains and the charge state of iron. Optical spectroscopic measurements were used to monitor the rate of change of oxidation state from rust-red ferric iron to colorless ferrous iron when irradiated by X-rays.
The I24 beamline team at Diamond Light Source helped design two X-ray experiments to expose the iron binding protein to specific X-ray doses. The experiments used a technique called serial crystallography, which sequentially exposes thousands of crystals briefly to the X-ray beam. These many single crystal measurements are then merged to form a complete, high-quality dataset.
Ivo Tews says, “Our work included many different types of experiments and sources, but one that stands out is serial synchrotron crystallography at Diamond Light Source that allowed us to follow changes in structure of FutA, in real time, under ambient conditions.”
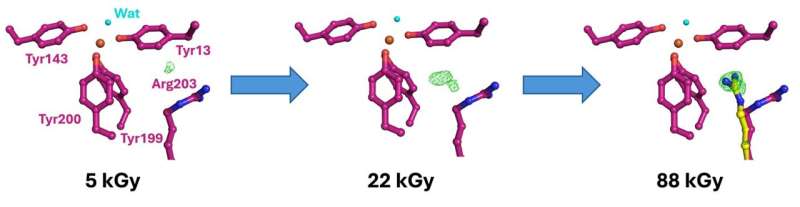
Changes in binding of iron in the iron binding protein FutA as revealed by dose-resolved serial synchrotron crystallography. The green difference density suggests that X-ray induced reduction causes the sidechain Arg203 to take an alternative conformation corresponding to iron (shown as a red sphere) transitioning from ferric to ferrous state. © Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2308478121
I24 has developed hardware for fixed target serial crystallography that can be used at both Diamond and at other sources such as the X-ray Free Electron Laser (XFEL) SACLA in Japan. To enable experiments at SACLA, I24 fixed target hardware was shipped to Japan and built up at the XFEL beamline. The experiments performed at SACLA allowed exposure to multiple, extremely brief X-ray pulses, while the second set of experiments performed at beamline I24 enabled multiple structures to be determined at accumulating dose.
The changing structure of the protein bound to iron before and after X-ray exposure revealed the changes the protein is required to make to perform its dual iron binding function. The use of identical hardware at both Diamond and SACLA simplifies sample preparation and reduces the risk associated with challenging experiments carried out halfway round the world.
Robin Owen, principal beamline scientist at I24, said, “Close collaboration between SACLA and Diamond to exploit the complementary nature of serial experiments at synchrotrons and free electron lasers was key to enabling insight into the dose response of FutA.”
The research probes how bacteria utilize iron, by using a protein specialized in iron binding with the surprise being that two different forms or oxidation states of iron can be bound by a single protein, FutA. This reflects two essential functions, namely the uptake of ferric iron from the environment, and the protection of the bacterial photosystems as ferrous iron.
As cyanobacteria typically have two types of proteins for these functions, it is speculated that the presence of a single protein in the cyanobacterium Prochlorococcus that can perform both tasks is an important factor in its ecological success.
More information:
Rachel Bolton et al, A redox switch allows binding of Fe(II) and Fe(III) ions in the cyanobacterial iron-binding protein FutA from Prochlorococcus, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2308478121
Provided by
Diamond Light Source
Citation:
How a cyanobacterium manages iron scarcity makes it the most successful photosynthetic organism on Earth (2024, April 17)