Plasmas are used in wound treatment against pathogens that are resistant to antibiotics. However, bacteria can defend themselves. They employ a heat shock protein that protects them.
A research team headed by Professor Julia Bandow and Dr. Tim Dirks from the Chair for Applied Microbiology at Ruhr University Bochum, Germany, showed that bacteria that overproduce the heat shock protein Hsp33 can withstand plasma treatment more effectively than others. The researchers also demonstrated which components of the plasma activate the heat shock protein. The team published their findings in the Journal of the Royal Society Interface.
All bacteria inactivated after three minutes
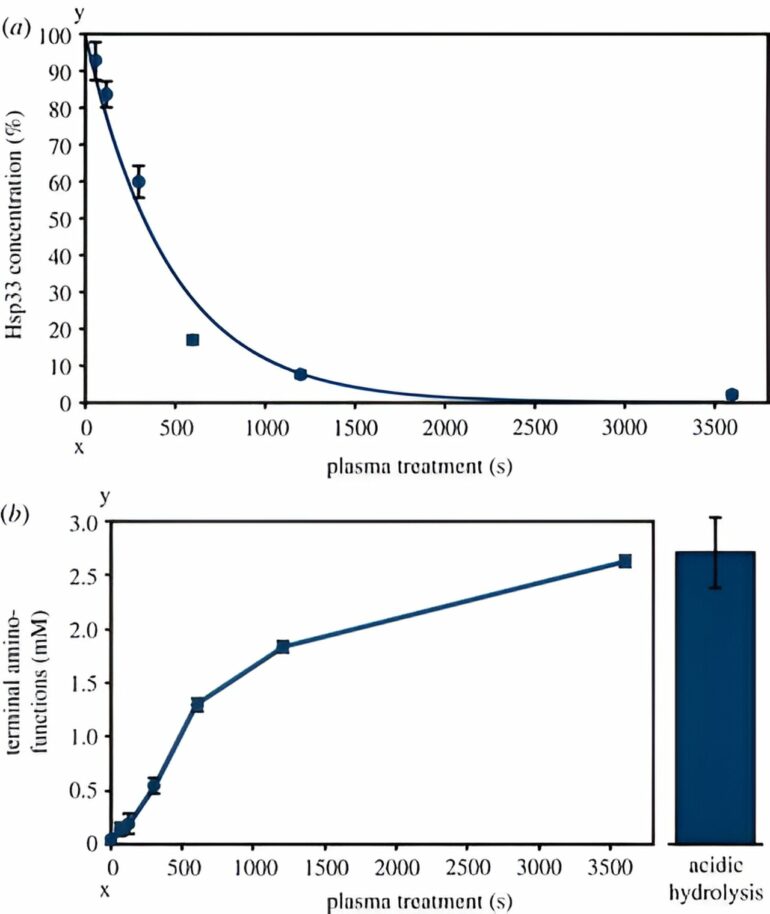
When treated with plasma, proteins unfold, lose their natural functions and can clump together. Their clumping is toxic to cells and can lead to their inactivation. The bacterial heat shock protein with a size of 33 kDa (kilo Dalton), referred to as Hsp33, prevents clumping by binding unfolding proteins.
To find out whether an excess of Hsp33 protects cells from plasma, the researchers treated strains that overproduce the protein with the Cinogy plasma source, which is already used in dermatology. These strains survived significantly better than wild-type bacteria after a short treatment of around one minute. “After a treatment of three minutes, the cells that produce Hsp33 in excess were also inactivated,” Tim Dirks points out.
Species that activate the heat shock protein
The researchers proved that Hsp33 is activated by plasma by treating the purified heat shock protein with the plasma source. “This activation is associated with the oxidation and unfolding of the protein and is actually reversible,” explains Tim Dirks. “However, we also showed that Hsp33 was completely degraded by longer plasma treatment times of one hour.” In addition, the protein’s ability to bind a zinc atom was adversely affected by plasma. This zinc atom strengthens the natural three-dimensional structure of the protein in its inactive state.
Since nothing was previously known as to which plasma-produced species can activate Hsp33, the researchers created various stressors that are known to be produced by plasma and treated Hsp33 with them one by one.
“This showed that Hsp33 is activated by superoxide, singular oxygen, and atomic oxygen, but doesn’t react to hydroxyl radicals and peroxynitrite,” says Tim Dirks.
This gives an indication of the interaction of these species with the bacterial cells. For example, superoxide is one of the first species generated by oxidative stress in the body, such as by our immune system in macrophages. A rapid response of Hsp33 to one of these species generated early on would therefore be advantageous for the bacterium for rapid protection against oxidative stress.
“Superoxide appears to act as a signaling molecule for bacteria, which signals further oxidative stress,” the research team concludes.
More information:
Tim Dirks et al, The cold atmospheric pressure plasma-generated species superoxide, singlet oxygen and atomic oxygen activate the molecular chaperone Hsp33, Journal of The Royal Society Interface (2023). DOI: 10.1098/rsif.2023.0300
Provided by
Ruhr-Universitaet-Bochum
Citation:
How bacteria defend themselves against plasmas (2023, November 24)