Food productivity is dependent on the availability of fertilizer, says Utah State University biochemist Lance Seefeldt. “We need nitrogen to survive, but we can’t take it in from the air,” says Seefeldt, professor and head of USU’s Department of Chemistry and Biochemistry.
“We get nitrogen from the protein in our food.”
Little over a century ago, the Haber-Bosch process revolutionized how atmospheric nitrogen could be converted to a form to allow for industrial-scale production of fertilizer.
The discovery led to a huge increase in global food production and a massive population boom. Still, certain areas of the globe, including Sub-Saharan Africa, lack the infrastructure to allow import and distribution of fertilizer, much less the capacity to produce the nutrient-essential product close to home.
Since 2019, Seefeldt and USU Senior Scientist Zhi-Yong Yang have collaborated on a project with colleagues in Spain and the United States to re-engineer the biology of cereal crops, such as corn and rice, to achieve nitrogen fixation on their own, from sunlight, without applying fertilizer.
With lead author Luis Rubio and Lucía Payá Tormo, Carlos Echavarri-Erasun, Natalia Makarovsky-Saavedra and Ana Pérez-González at the Center for Plant Biotechnology and Genomics at the Polytechnic University of Madrid (UPM), along with Yisong Guo of Carnegie Mellon University, Seefeldt and Yang report a simpler pathway, involving a newly known minimum of seven genes that allow the plant cell to make the enzyme that can covert N2 gas from the air to fertilizer.
Their findings appear in the Nov. 6, 2024 issue of the Proceedings of the National Academy of Sciences.
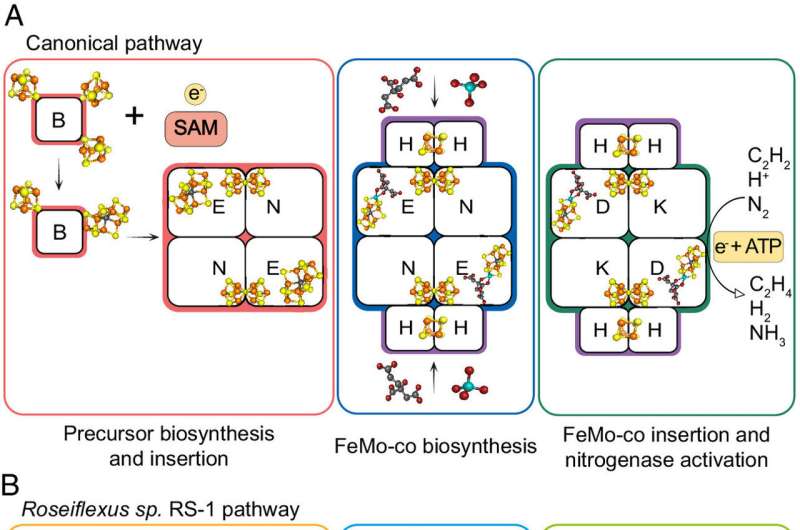
Schemes of FeMo-co biosynthetic pathways. © Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2406198121
“The goal is to place genes into the crops’ mitochondria and chloroplast, enabling them to generate sufficient energy to drive nitrogen fixation,” Yang says. “This is a pretty cool piece of evidence. Essentially, these staple caloric crops—rice, corn, potatoes—could have built-in fertilizer.”
The team initially narrowed the number of genes needed to fix nitrogen to nine, and identified gene combinations they thought were essential to complete the process. To their surprise, they discovered genes they’d designated as critical middlemen could be omitted.
The possibility of freeing cereal crops from the need for added fertilizer is significant, Seefeldt says.
“Haber-Bosch has prevented mass starvation and enables most of us to enjoy a bountiful, stable food supply, but it carries a heavy carbon footprint,” he says. “Nearly 2% of the world’s fossil fuel supply goes toward fertilizer production, which is highly pollutive.”
Further, the newly discovered minimum genes could help to alleviate hunger in less-developed, less-accessible parts of the world, as well as areas increasingly plagued by climate-driven drought.
The knowledge also advances research focused on food production beyond Earth. Seefeldt and USU colleague Bruce Bugbee have collaborated on efforts investigating how to sustain human life on long-duration space missions, including trips to Mars.
“Piece by piece, we’re learning what genes and what combination of genes are needed to achieve nitrogen fixation in different cells,” Seefeldt says. “Instead of just one horn playing, we’re trying to get the whole orchestra to play together.”
More information:
Lucía Payá-Tormo et al, Iron-molybdenum cofactor synthesis by a thermophilic nitrogenase devoid of the scaffold NifEN, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2406198121
Provided by
Utah State University
Citation:
New research could simplify genetic transfer of nitrogen fixation to food crops (2024, November 8)



