According to the World Health Organization, enterotoxigenic Escherichia coli (ETEC) bacteria cause the largest number of recorded community-acquired cases of childhood diarrhea in the developing world and is the most common culprit in traveler’s diarrhea. While in healthy adults this is merely an unpleasant inconvenience, in infants and young children this can lead to chronic malnutrition, stunted growth and impaired cognitive function.
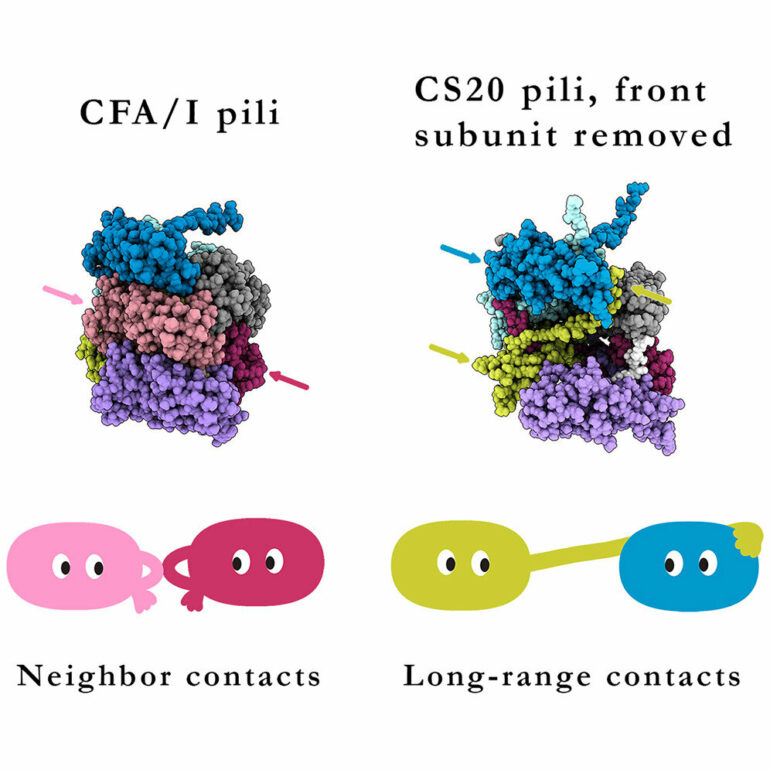
ETEC uses long, thin filaments called ‘pili’ to attach to host intestinal epithelia, an early, vital step in diarrhea pathogenesis. These fibers are essential for initiation of infection in the intestines by ETEC. Now, researchers from Boston University Chobanian & Avedisian School of Medicine have found that pili are fine-tuned for their preferred microenvironment, such as the gut or the urinary tract. They also found some use short loops to bind themselves to one another. Others use long extensions to stretch a long way. This discovery has important implications for creating better therapeutics against diarrheal diseases.
“Unwinding (and rewinding) of pili reduces the force at the site of binding, and allows the bacteria to stay attached. Because pili are critical for bacteria to cause disease, finding a way to prevent pili from unwinding and rewinding can be used in the future to prevent diarrheal disease,” explained corresponding author Esther Bullitt, Ph.D., associate professor of physiology & biophysics.
The researchers used a combination of methods that included imaging the pili in a near-native state using a cryogenic electron microscope, using lasers to pull on the pili and measure the force it takes to unwind them and computer simulations to watch the unwinding on the atomic level.
Because pili on different strains of bacteria are tuned for their environment, the researchers are looking for ways to specifically target disease-causing bacteria. “Therapeutics that disrupt pili and allow bacteria to be washed away have advantages over current antibiotics, as physical removal would not lead to the evolution of resistant strains and only the pathogens would be targeted, while leaving the ‘good’ bacteria of the microbiome intact,” said Bullitt.
These finds appear online in the journal Structure.
More information:
Esther Bullitt, Three structural solutions for bacterial adhesion pilus stability and superelasticity, Structure (2023). DOI: 10.1016/j.str.2023.03.005. www.cell.com/structure/fulltex … 0969-2126(23)00081-3
Provided by
Boston University School of Medicine
Citation:
Researchers solve the cell structure responsible for traveler’s diarrhea (2023, March 30)