Scientists have discovered a revolutionary way to put an end to jet lag by uncovering the secret at the tail end of Casein Kinase 1 delta (CK1δ), a protein that regulates our body clock. This breakthrough, achieved by researchers from Duke-NUS Medical School and the University of California, Santa Cruz, offers a new approach to adjusting our circadian rhythms, the natural 24-hour cycles that influence sleep-wake patterns and overall daily functions.
Published in the journal Proceedings of the National Academy of Sciences (PNAS), their findings could pave the way for new approaches to treating disorders related to the body clock.
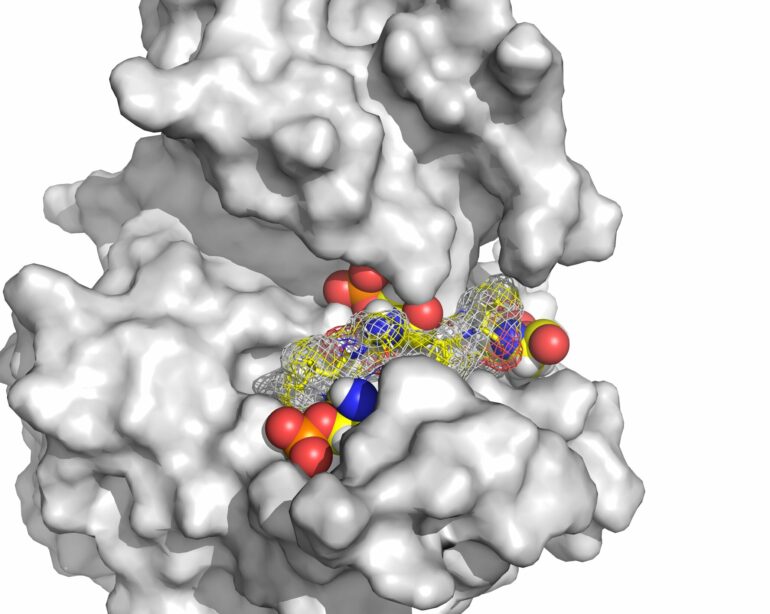
CK1δ regulates circadian rhythms by tagging other proteins involved in our biological clock to fine-tune the timing of these rhythms. In addition to modifying other proteins, CK1δ itself can be tagged, thereby altering its own ability to regulate the proteins involved in running the body’s internal clock.
Previous research identified two distinct versions of CK1δ, known as isoforms δ1 and δ2, which vary by just 16 building blocks or amino acids right at the end of the protein in a part called the C-terminal tail. Yet these small differences significantly impact CK1δ’s function. While it was known that when these proteins are tagged, their ability to regulate the body clock decreases, no one knew exactly how this happened.
Using advanced spectroscopy and spectrometry techniques to zoom in on the tails, the researchers found that how the proteins are tagged is determined by their distinct tail sequences.
Howard Hughes Medical Institute Investigator Professor Carrie Partch from the Department of Chemistry & Biochemistry at the University of California, Santa Cruz and corresponding author of the study explained:
“Our findings pinpoint to three specific sites on CK1δ’s tail where phosphate groups can attach, and these sites are crucial for controlling the protein’s activity. When these spots get tagged with a phosphate group, CK1δ becomes less active, which means it doesn’t influence our circadian rhythms as effectively. Using high-resolution analysis, we were able to pinpoint the exact sites involved—and that’s really exciting.”
Having first studied this protein more than 30 years ago while investigating its role in cell division, Professor David Virshup, the director of the Cancer and Stem Cell Biology Program at Duke-NUS and co-corresponding author of the study, said, “With the technology we have available now, we were finally able to get to the bottom of a question that has gone unanswered for more than 25 years.
“We found that the δ1 tail interacts more extensively with the main part of the protein, leading to greater self-inhibition compared to δ2. This means that δ1 is more tightly regulated by its tail than δ2. When these sites are mutated or removed, δ1 becomes more active, which leads to changes in circadian rhythms. In contrast, δ2 does not have the same regulatory effect from its tail region.”
This discovery highlights how a small part of CK1δ can greatly influence its overall activity. This self-regulation is vital for keeping CK1δ activity balanced, which, in turn, helps regulate our circadian rhythms.
The study also addressed the wider implications of these findings. CK1δ plays a role in several important processes beyond circadian rhythms, including cell division, cancer development, and certain neurodegenerative diseases. By better understanding how CK1δ’s activity is regulated, scientists could open new avenues for treating not just circadian rhythm disorders but also a range of conditions.
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, added, “Regulating our internal clock goes beyond curing jet lag—it’s about improving sleep quality, metabolism and overall health. This important discovery could potentially open new doors for treatments that could transform how we manage these essential aspects of our daily lives.”
The researchers plan to further investigate how real-world factors, such as diet and environmental changes, affect the tagging sites on CK1δ. This could provide insights into how these factors affect circadian rhythms and might lead to practical solutions for managing disruptions.
More information:
Rachel L. Harold et al, Isoform-specific C-terminal phosphorylation drives autoinhibition of Casein kinase 1, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2415567121
Provided by
Duke-NUS Medical School
Citation:
Ending jet lag: Scientists discover secret to regulating our body clock (2024, October 7)