Researchers from Tokyo Metropolitan University have made strides forward in realizing industrial conversion of bicarbonate solution made from captured carbon to a formate solution, a green fuel. The research is published in the journal EES Catalysis.
Their new electrochemical cell, with a porous membrane layer in between the electrodes, overcomes major issues suffered in reactive carbon capture (RCC) and achieves performances rivaling energy-hungry gas-fed methods. Processes like theirs directly add value to waste streams and are key to realizing net zero emissions.
Carbon capture technology is a big part of the global strategy to reduce emissions and fight climate change. But the important question of what we do with the captured carbon dioxide remains an open challenge. Do we simply push it underground, or is there more to it? Scientists certainly think so. Using state-of-the-art catalysts and chemical processes, work is under way to try and convert the captured product into something more useful for society.
One particularly enticing application is the conversion of carbon dioxide into an environmentally-friendly fuel. Technology has been developed for using electrochemical cells to reduce the carbon dioxide to a formate compound, which itself can be used in formate fuel cells to generate power.
However, a significant roadblock is the need for pure carbon dioxide: pressurizing carbon dioxide can be highly energy intensive. The gas is not converted very efficiently, and the cells do not last very long. Enter reactive carbon capture, where carbon dioxide dissolved in alkaline solutions, like bicarbonate solutions, can be directly used to create formate ions without the losses associated with providing pure gas.
The key challenge facing researchers here is the design of a better electrochemical cell which can selectively produce formate ions from bicarbonate ions without losing out to side reactions, like the production of hydrogen.
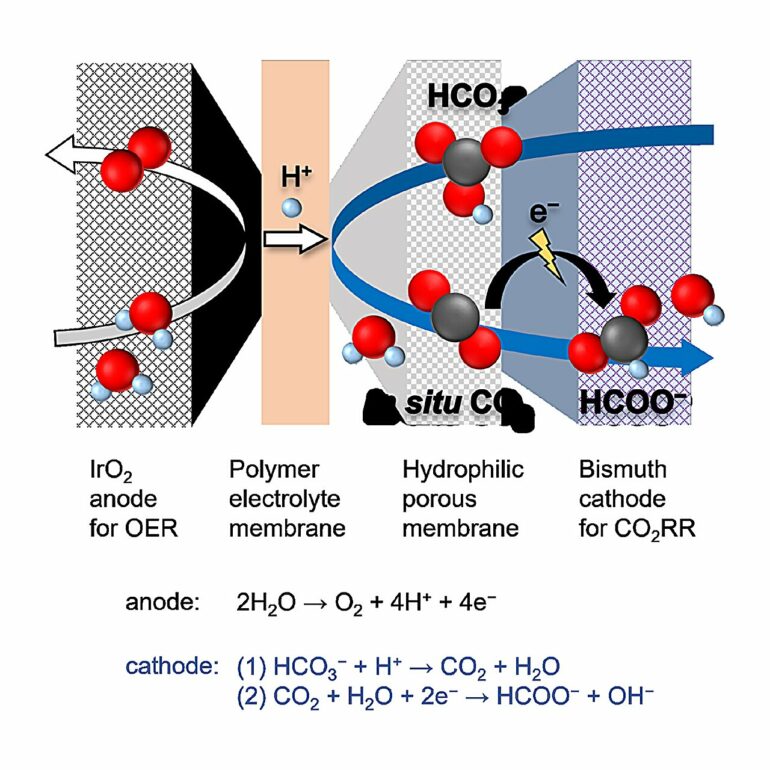
Now, a team of researchers led by Professor Fumiaki Amano from Tokyo Metropolitan University have created a new cell with excellent selectivity for the conversion of bicarbonate ions into formate ions. In the new cell, electrodes made of catalytic material are separated from a polymer electrolyte membrane by a porous membrane made of cellulose ester.
Hydrogen ions produced at one electrode pass through the electrolyte membrane and make it to the porous layer, where they react with bicarbonate ions to efficiently produce carbon dioxide in the pores. The gas is then converted to formate ions at the other electrode, also in contact with the porous membrane.
When they put their cell to work, they found that the faradaic efficiency of their cell, the proportion of electrons converted to formate instead of other compounds, was 85%, even with very high currents. Not only does this outperform existing designs, the cell was found to operate smoothly for over 30 hours and realize nearly complete conversion of bicarbonate to formate. Once the water has been driven off, all that is left is solid, crystalline formate fuel.
Given the demands for climate change technology, improvements like this to the efficient running of electrochemical cells promise to have a big impact. The team hopes their new bicarbonate electrolyzer can be a viable option for society as it strives towards a green transformation.
More information:
Kohta Nomoto et al, Highly selective formate formation via bicarbonate conversions, EES Catalysis (2024). DOI: 10.1039/D4EY00122B
Provided by
Tokyo Metropolitan University
Citation:
Scientists reveal new electrochemical cell design for turning carbon dioxide into a green fuel (2024, September 16)