Chemical reactions and physical processes that involve water surfaces will be easier to model thanks to the discovery by an all-RIKEN team of how the molecules at water surfaces lose energy.
Water is anomalous in many ways. For example, it has far higher freezing and boiling points than would be expected based on a naive comparison with other hydrides. Most of these anomalous properties stem from the strong electrostatic pull that a hydrogen atom feels for an oxygen atom in a nearby molecule. This attractive force gives rise to hydrogen bonds between neighboring water molecules.
These hydrogen bonds form a 3D network within a body of water. But the layer of molecules lying at the surface of the water differs from the other molecules in that it forms hydrogen bonds only with molecules lying below it. Because this layer is only one molecule thick, not much is known about it.
Now, Tahei Tahara of the RIKEN Molecular Spectroscopy Laboratory and his co-workers have discovered how these surface molecules lose energy.
“Water interfaces play key roles in many fundamental chemical and physical processes,” says Tahara. “So understanding how interfacial water dissipates energy is crucial for understanding and controlling interfacial phenomena at the molecular level.”
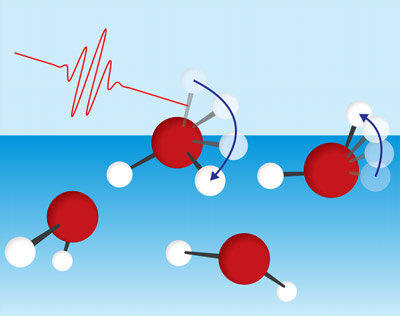
Surface water molecules have one hydroxyl group (OH) sticking out into the air, which is free from the hydrogen-bond network. The team discovered that surface water molecules mainly dissipate energy by rotating this protruding OH bond (Fig. 1). This goes against conventional wisdom, namely that the surface molecules lose energy only through interacting with neighboring molecules.
“This finding completely goes against the existing belief that energy dissipation of the free OH proceeds with energy transfer,” notes Tahara.
This discovery will shed light on the dynamics of more than just water surfaces. “We believe that our finding provides a basis for fully elucidating dynamical processes including chemical reactions that proceed at the water interface,” says Tahara.
To make their discovery, the team used a spectroscopic technique that singles out the surface molecules and probes how they change with time. It was a challenging measurement to make. “This was a difficult and delicate experiment to perform,” says Tahara. “We had to detect femtosecond changes in the faint signal generated from just a single water layer at the air–water interface while controlling the phase of the femtosecond laser pulses.”
The team next intends to investigate how the hydrogen-bonded OH groups of interfacial water transfer energy. “This will give us a coherent and consistent view of energy-transfer processes at water interfaces,” says Tahara.
The water surface is a fantastic place for chemical reactions
More information:
Ken-ichi Inoue et al. Reorientation-induced relaxation of free OH at the air/water interface revealed by ultrafast heterodyne-detected nonlinear spectroscopy, Nature Communications (2020). DOI: 10.1038/s41467-020-19143-8
Citation:
Water surface molecules lose energy through rotation of free OH group (2021, April 2)
retrieved 4 April 2021
from https://phys.org/news/2021-04-surface-molecules-energy-rotation-free.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.