The genetic plans within our DNA come to functional fruition through proteins, which underlie our bodies’ structure and activity. Yet, the proteome—all the proteins within a cell or given area—remains relatively mysterious because protein landscapes are incredibly complex. Humans, for example, make tens of thousands of different proteins.
To help decipher this complexity, a team of Stanford University researchers has led the development of a new method, called TransitID, for tracking the complete activity of proteins in living cells. This method is detailed in a paper published June 28 in the journal Cell.
Existing techniques for this task, microscopy, and mass spectrometry proteomics, either allow scientists to study only a handful of proteins at a time in a live cell, or provide a very detailed, but still, snapshot of all the proteins in a dead cell.
“Our new technique allows you to combine the strengths of both microscopy and mass spectrometry proteomics by actually looking at living samples—including their dynamics as they move and function—and seeing in an unbiased way all of the proteins at once,” said Alice Ting, professor of genetics at Stanford Medicine and of biology in the School of Humanities and Sciences, who is senior author of the paper. “There haven’t been methods that combined those strengths before.”
TransitID also works on proteins that move between cells. Tracking proteins at this level of detail and liveliness could unveil untold information about how cells communicate. In addition, there are obvious applications for research into various diseases and treatments, including in the realms of cancer and neurodegenerative diseases.
“It’s exciting that there are such broad applications for this,” said Joleen Cheah, a Ph.D. candidate at Stanford and co-lead author of the paper. “We’ve already received many inquiries about TransitID and established many collaborations with other labs that are interested in using it.”
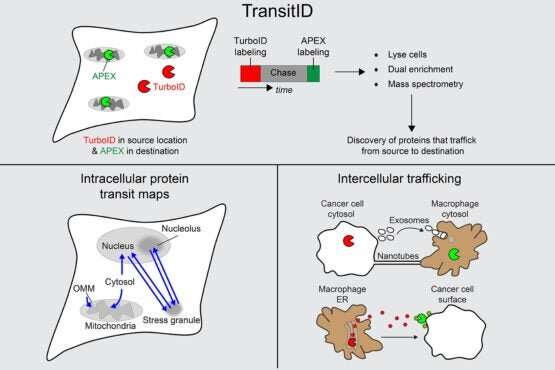
TransitID workflow and applications to cell biology and cancer. © Joleen Cheah
Tracing a collective journey
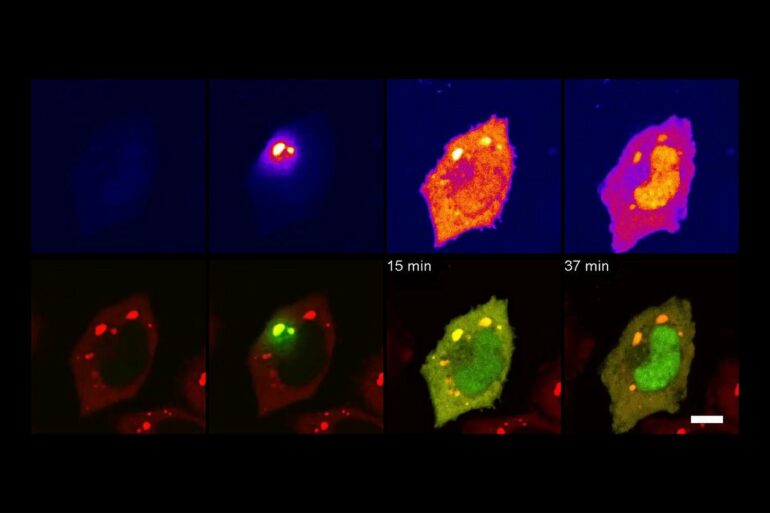
In very simple terms, TransitID tracks all the proteins along a specific journey by tagging all the molecules within a certain radius of chosen beginning and end locations. First, two enzymes developed previously by the Ting lab, called TurboID and APEX, are placed at each end of the journey. When the researchers are ready to begin their protein monitoring, they introduce the B vitamin biotin, which causes TurboID to spray biotin on all the surrounding molecules—including proteins—tagging them.
The researchers then wash the cell of excess biotin and allow it to go about its usual activity. When they think the proteins have had enough time to travel, they then add the chemical compound alkyne-phenol, which causes the same spraying action on the APEX end.
The researchers interpret the individual excursions of the proteins that moved between the tagged locations by breaking down the cell membrane and analyzing the contents. Some proteins will never have moved, either having only TurboID tags or only APEX tags. Those that have both, made the journey between. And anything without a tag resided beyond the journey of interest.
Four experiments, one surprise
In order to test their tool, the researchers first conducted two experiments detailing known protein activity. They monitored proteins that moved between the fluid of the cytoplasm (the cytosol) to the mitochondria, which exist in contrast to proteins that are the product of mitochondrial DNA and therefore originate in the mitochondria.
In the next experiment, the researchers tracked how protein movement from the cytosol to the nucleus changes when a cell undergoes oxidative stress. The results from both of these experiments turned out as expected, which meant TransitID was working successfully.
Next, the researchers used their technique to investigate protein traffic to parts of the cell that are not bound by membranes—specifically, stress granules, which are condensations of proteins and RNAs that form in response to cellular stress.
“Biomolecular condensates, such as stress granules, are highly dynamic structures that rapidly and continually exchange constituents with related structures. TransitID allows us for the first time to track the fate of these constituents,” said J. Paul Taylor, scientific director and executive vice president at St. Jude Children’s Research Hospital, who is co-author of the paper.
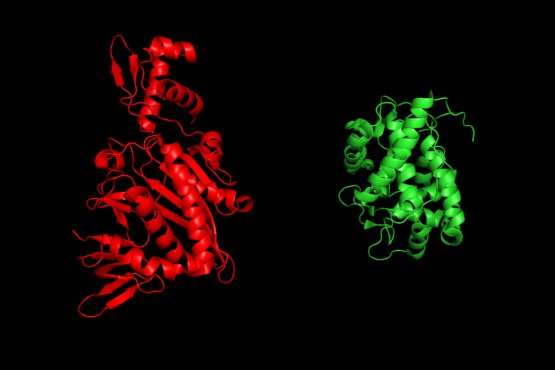
Protein structures of proximity labeling enzymes, TurboID (left) and APEX (right). © Joleen Cheah
The results of this experiment were surprising. As the researchers were analyzing the proteome of the stress granules, they found JUN, a well-known transcription factor that is upregulated/overexpressed in multiple cancers.
“No one has ever found it in stress granules before,” said Ting. “So, when we found it there, we almost didn’t trust it.”
After confirming its presence, the researchers probed further and determined that JUN seemed to be in the stress granule as a protection mechanism. They found that, under oxidative stress, JUN relocates to the stress granule to avoid its alternative stress response—aggregating and then being disposed of for being an unwelcome aggregated mess—which keeps JUN healthy and available to resume its work once the stress is over.
The last experiment with TransitID included in this paper tracked protein activity between macrophages and cancer cells, which exists in a notoriously complex and noisy protein environment.
Improving on great potential
With thousands of requests for TurboID and APEX already received from labs around the world in response to previous work, the researchers know their new take on these existing favorites is bound to enable thrilling results in both foundational and applied science.
“I expect this approach to be rapidly adopted by a broad array of scientists, similar to other innovative techniques developed in Alice’s lab,” said Taylor. “This tool is a particularly important breakthrough enabling the investigation of condensates because they are highly dynamic structures.”
“I’m excited about the simplicity of the method and how accessible it is, given what’s already out there,” said Ting. “It uses commercially available reagents and people don’t have to do any organic chemistry in their own labs to use the method, and yet they can access totally new biology that wasn’t visible before.”
The team still sees room for improvement, specifically aiming to optimize their process and rely on less toxic chemicals. For now, TransitID is limited to being used in cell cultures but, with gentler chemicals, it could be used to track detailed protein dynamics in living animals.
More information:
Wei Qin et al, Dynamic mapping of proteome trafficking within and between living cells by TransitID, Cell (2023). DOI: 10.1016/j.cell.2023.05.044
Provided by
Stanford University
Citation:
Tagging system allows researchers to track protein traffic in living cells (2023, June 29)