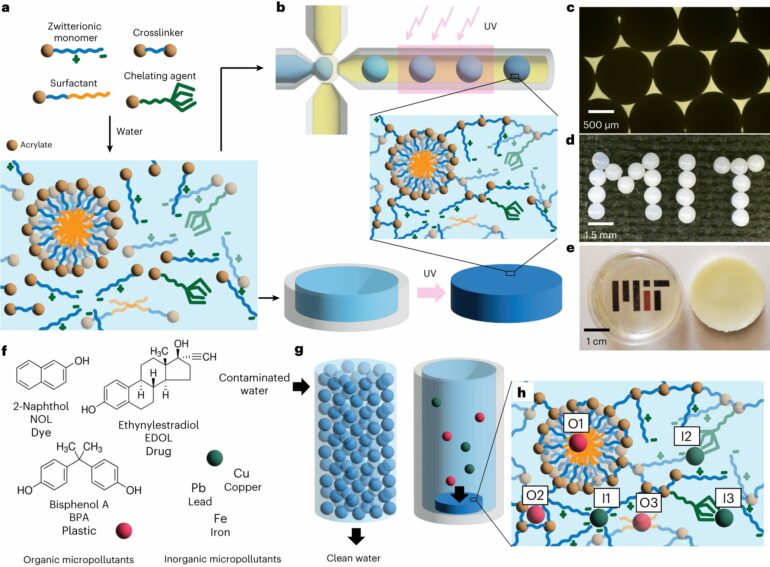
“Zwitterionic” might not be a word you come across every day, but for Professor Patrick Doyle of the MIT Department of Chemical Engineering, it’s a word that’s central to the technology his group is developing to remove micropollutants from water. Derived from the German word “zwitter,” meaning “hybrid,” “zwitterionic” molecules are those with an equal number of positive and negative charges.
Devashish Gokhale, a Ph.D. student in Doyle’s lab, uses the example of a magnet to describe zwitterionic materials: “On a magnet, you have a north pole and a south pole that stick to each other, and on a zwitterionic molecule, you have a positive charge and a negative charge which stick to each other in a similar way.”
Because many inorganic micropollutants and some organic micropollutants are themselves charged, Doyle and his team have been investigating how to deploy zwitterionic molecules to capture micropollutants in water.
In a new paper in Nature Water, Doyle, Gokhale, and undergraduate student Andre Hamelberg explain how they use zwitterionic hydrogels to sustainably capture both organic and inorganic micropollutants from water with minimal operational complexity. In the past, zwitterionic molecules have been used as coatings on membranes for water treatment because of their non-fouling properties. But in the Doyle group’s system, zwitterionic molecules are used to form the scaffold material, or backbone within the hydrogel—a porous three-dimensional network of polymer chains that contains a significant amount of water.
“Zwitterionic molecules have very strong attraction to water compared to other materials which are used to make hydrogels or polymers,” says Gokhale. What’s more, the positive and negative charges on zwitterionic molecules cause the hydrogels to have lower compressibility than what has been commonly observed in hydrogels. This makes for significantly more swollen, robust, and porous hydrogels, which is important for the scale up of the hydrogel-based system for water treatment.
Seeking a sustainable solution
Micropollutants are chemically diverse materials that can be harmful to human health and the environment, even though they are typically found at low concentrations (micrograms to milligrams per liter) relative to conventional contaminants. Micropollutants can be organic or inorganic and can be naturally-occurring or synthetic. Organic micropollutants are mostly carbon-based molecules and include pesticides and per- and polyfluoroalkyl substances (PFAS), known as “forever chemicals.” Inorganic micropollutants, such as heavy metals like lead and arsenic, tend to be smaller than organic micropollutants. Unfortunately, both organic and inorganic micropollutants are pervasive in the environment.
Many micropollutants come from industrial processes, but the effects of human-induced climate change are also contributing to the environmental spread of micropollutants. Gokhale explains that in California, for example, fires burn plastic electrical cables and leech micropollutants into natural ecosystems.
Doyle adds that “outside of climate change, things like pandemics can spike the number of organic micropollutants in the environment due to high concentrations of pharmaceuticals in wastewater.”
It’s no surprise, then, that over the past few years, micropollutants have become more and more of a concern. These chemicals have garnered attention in the media and led to “significant change in the environmental engineering and regulatory landscape,” says Gokhale.
In March 2023, the U.S. Environmental Protection Agency (EPA) proposed a strict, federal standard that would regulate six different PFAS chemicals in drinking water. Just last October, the EPA proposed banning the micropollutant trichloroethylene, a cancer-causing chemical that can be found in brake cleaners and other consumer products. And as recently as November, the EPA proposed that water utilities nationwide be required to replace all of their lead pipes to protect the public from lead exposure.
Internationally, Gokhale notes the Oslo Paris Convention, whose mission is to protect the marine environment of the northeast Atlantic Ocean, including phasing out the discharge of offshore chemicals from the oil and gas industries.
With each new, necessary regulation to protect the safety of our water resources, the need for effective water treatment processes grows. Compounding this challenge is the need to make water treatment processes that are sustainable and energy-efficient.
The benchmark method to treat micropollutants in water is activated carbon. However, making filters with activated carbon is energy-intensive, requiring very high temperatures in large, centralized facilities. Gokhale says that approximately “four kilograms of coal are needed to make one kilogram of activated carbon, so you lose a significant amount of carbon dioxide to the environment.” According to the World Economic Forum, global water and wastewater treatment accounts for 5% of annual emissions. In the U.S. alone, the EPA reports that drinking water and wastewater systems account for over 45 million tons of greenhouse gas emissions annually.
“We need to develop methods which have smaller climate footprints than methods which are being used industrially today,” says Gokhale.
Supporting a ‘high-risk’ project
In September 2019, Doyle and his lab embarked on an initial project to develop a microparticle-based platform to remove a broad range of micropollutants from water. His group had been using hydrogels in pharmaceutical processing to formulate drug molecules into pill format. He soon realized his pharmaceutical work with hydrogels could be applied to environmental issues like water treatment.
In March 2022, Doyle, Gokhale, and MIT undergraduate Ian Chen published findings describing their use of micelles within hydrogels for water treatment. Micelles are spherical structures that form when molecules called surfactants (found in things like soap), come in contact with water or other liquids.
The team was able to synthesize micelle-laden hydrogel particles that soak up micropollutants from water like a sponge. Unlike activated carbon, the hydrogel particle system is made from environmentally friendly materials. Furthermore, the system’s materials are made at room temperature, making them exceedingly more sustainable than activated carbon.
Building off that success, Doyle and his team began in September 2022 to move their technology from the lab to the market. They have been able to build, test, and refine pilot-scale prototypes of their hydrogel platform. System iterations have included the use of the zwitterionic molecules, a novel advancement from their earlier work.
Rapid elimination of micropollutants is of special importance in commercial water treatment processes, where there is a limited amount of time water can spend inside the operational filtration unit. This is referred to as contact time, explains Gokhale. In municipal-scale or industrial-scale water treatment systems, contact times are usually less than 20 minutes and can be as short as five minutes.
“But as people have been trying to target these emerging micropollutants of concern, they realized they can’t get to sufficiently low concentrations on the same time scales as conventional contaminants,” Gokhale says.
“Most technologies focus only on specific molecules or specific classes of molecules. So, you have whole technologies which are focusing only on PFAS, and then you have other technologies for lead and metals. When you start thinking about removing all of these contaminants from water, you end up with designs which have a very large number of unit operations. And that’s an issue because you have plants which are in the middle of large cities, and they don’t necessarily have space to expand to increase their contact times to efficiently remove multiple micropollutants,” he adds.
Since zwitterionic molecules possess unique properties that confer high porosity, the researchers have been able to engineer a system for quicker uptake of micropollutants from water. Tests show that the hydrogels can eliminate six chemically diverse micropollutants at least 10 times faster than commercial activated carbon.
The system is also compatible with a diverse set of materials, making it multifunctional. Micropollutants can bind to many different sites within the hydrogel platform: organic micropollutants bind to the micelles or surfactants while inorganic micropollutants bind to the zwitterionic molecules. Micelles, surfactants, zwitterionic molecules, and other chelating agents can be swapped in and out to essentially tune the system with different functionalities based on the profile of the water being treated.
This kind of “plug-and-play” addition of various functional agents does not require a change in the design or synthesis of the hydrogel platform, and adding more functionalities does not take away from existing functionality. In this way, the zwitterionic-based system can rapidly remove multiple contaminants at lower concentrations in a single step, without the need for large, industrial units or capital expenditure.
Perhaps most importantly, the particles in the Doyle group’s system can be regenerated and used over and over again. By simply soaking the particles in an ethanol bath, they can be washed of micropollutants for indefinite use without loss of efficacy. When activated carbon is used for water treatment, the activated carbon itself becomes contaminated with micropollutants and must be treated as toxic chemical waste and disposed of in special landfills. Over time, micropollutants in landfills will reenter the ecosystem, perpetuating the problem.
Arjav Shah, a Ph.D.-MBA candidate in MIT’s Department of Chemical Engineering and the MIT Sloan School of Management, respectively, recently joined the team to lead commercialization efforts. The team has found that the zwitterionic hydrogels could be used in several real-world contexts, ranging from large-scale industrial packed beds to small-scale, portable, off-grid applications—for example, in tablets that could clean water in a canteen—and they have begun piloting the technology through a number of commercialization programs at MIT and in the greater Boston area.
The combined strengths of each member of the team continue to drive the project forward in impactful ways, including undergraduate students like Andre Hamelberg, the third author on the Nature Water paper. Hamelberg is a participant in MIT’s Undergraduate Research Opportunities Program (UROP). Gokhale, who is also an Abdul Latif Jameel World Water and Food Security Lab (J-WAFS) Fellow, provides training and mentorship to Hamelberg and other UROP students in the lab.
“We see this as an educational opportunity,” says Gokhale, noting that the UROP students learn science and chemical engineering through the research they conduct in the lab. The project has also been “a way of getting undergrads interested in water treatment and the more sustainable aspects of chemical engineering,” Gokhale says. He adds that it’s “one of the few projects which goes all the way from designing specific chemistries to building small filters and units and scaling them up and commercializing them. It’s a really good learning opportunity for the undergrads and we’re always excited to have them work with us.”
In four years, the technology has been able to grow from an initial idea to a technology with scalable, real-world applications, making it an exemplar J-WAFS project. The fruitful collaboration between J-WAFS and the Doyle lab serves as inspiration for any MIT faculty who may want to apply their research to water or food systems projects.
“The J-WAFS project serves as a way to demystify what a chemical engineer does,” says Doyle. “I think that there’s an old idea of chemical engineering as working in just oil and gas. But modern chemical engineering is focused on things which make life and the environment better.”
More information:
Devashish Gokhale et al, Multifunctional zwitterionic hydrogels for the rapid elimination of organic and inorganic micropollutants from water, Nature Water (2024). DOI: 10.1038/s44221-023-00180-8
Provided by
Massachusetts Institute of Technology
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.
Citation:
Engineers create a zwitterionic hydrogel system to swiftly eliminate micropollutants from water (2024, January 9)