On a mission to build better electric vehicle batteries, chemists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have used an electrolyte additive to improve the functionality of energy-dense lithium metal batteries. By adding a compound called cesium nitrate to the electrolyte that separates the battery’s anode and cathode, the research team has significantly improved the charging rate of lithium metal batteries while maintaining a long cycle life.
The team’s new work, recently published in Nature Communications, targets the interphase—a protective layer formed on the battery’s anode and cathode. This layer, which prevents degradation of battery electrodes, is the key to creating lithium metal batteries that can be charged and discharged as many times as lithium-ion batteries.
“We wanted to improve the charging rate of the current state-of-the-art lithium metal batteries,” explained Muhammad Mominur Rahman, a research associate in the Electrochemical Energy Storage Group of the Chemistry Division at Brookhaven and first author on the new paper. “But we also wanted to stabilize the batteries with a more protective interphase so they would last longer.”
In addition to successfully stabilizing the battery, Rahman’s electrolyte additive altered the battery chemistry in an unexpected way.
“Mominur’s findings challenge conventional beliefs about the components of an effective interphase,” said Enyuan Hu, Brookhaven chemist and principal investigator within the Electrochemical Energy Storage Group. “We’re excited to see how these findings contribute to the major DOE effort focused on lithium metal batteries.”
From left to right: Brookhaven beamline scientist Sanjit Ghose with chemists Enyuan Hu and Muhammad Mominur Rahman at the National Synchrotron Light Source II X-ray Powder Diffraction beamline. © Jessica Rotkiewicz/Brookhaven National Laboratory
One step toward a larger goal
Hu and his team are working among other battery experts as part of the Battery500 Consortium, a collaboration of several national labs and universities. The Consortium, which is led by DOE’s Pacific Northwest National Laboratory, is striving to make batteries with an energy density of 500 watt-hours per kilogram—more than double the energy density of today’s state-of-the-art batteries.
This energy density cannot be achieved in the lithium-ion batteries powering most of today’s battery-operated devices—including phones, television remotes, and even electric vehicles. So, scientists needed to turn to lithium metal batteries to pursue their goals. These batteries possess a lithium metal anode, rather than the graphite anode present in lithium-ion batteries.
“The lithium metal battery is attractive because it can give twice the energy density of a battery with a graphite anode,” explained Rahman. “But there are lots of challenges to tackle.”
Brookhaven’s most recent research addresses one of these challenges—striking a balance between the charging speed and the cycle life.
The electrolyte that typically enables fast battery charging is also likely to be reactive with the lithium metal anode. If these chemical reactions proceed uncontrollably, the electrolyte decomposes and reduces the battery’s cycle life. To prevent this from happening, Brookhaven chemists set out to engineer the interphase.
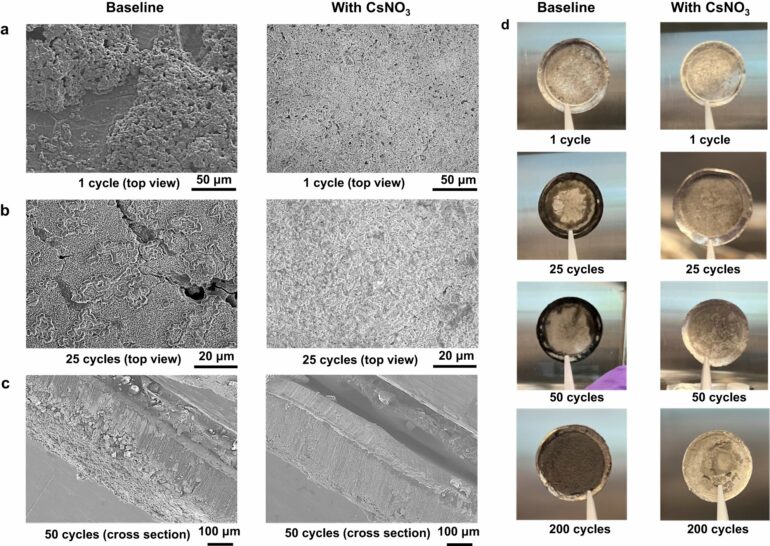
Previous studies had indicated that the lithium metal anode could be stabilized with a cesium additive. But to increase the charging rate while maintaining the battery cycle life, the anode and cathode need to be stabilized simultaneously. The Brookhaven scientists believed cesium nitrate could serve this purpose for lithium metal batteries. As they had hypothesized, the positive cesium ion accumulated on the negatively charged lithium metal anode side of the battery, while the negative nitrate ion accumulated on the positively charged cathode.
To better understand how the cesium nitrate additive influenced the electrolyte composition and battery performance, the chemists brought the new batteries to the National Synchrotron Light Source II (NSLS-II), a DOE Office of Science user facility at Brookhaven Lab.
A gaze into the interphase
NSLS-II is one of the most advanced X-ray light sources in the world, producing light beams that are 10 billion times brighter than the sun. Of the 29 beamlines currently operating at NSLS-II, Rahman and Hu took advantage of the capabilities of four beamlines for their most recent research.
“NSLS-II is really a great facility for conducting battery research,” said Hu. “There is a breadth of techniques available, which enables us to conduct complete studies of complex materials.”
Among the four beamlines used by the chemists was the X-ray Powder Diffraction (XPD) beamline, a high energy diffraction beamline with photon beams that can contain more than three times the energy of conventional X-ray powder diffraction beamlines. For more than five years, Hu’s group has been leveraging these high energy beams for interphase studies that have led to a series of new understandings of battery chemistry.
The high-energy X-rays are capable of penetrating thick materials, like the anodes and cathodes within batteries. But they are also characterized by their high intensity, which enables the quick data collection necessary to take a “snapshot” of the elusive interphase.
“The XPD beamline is excellent because its X-rays have low absorption power and do not damage the interphase samples,” Hu elaborated. “One of the greatest challenges in characterizing interphase samples is their sensitivity to the X-ray beams, but we’ve characterized over 1,000 interphase samples at XPD without observing any damage to the samples.”
Some components of the interphase are crystalline, meaning that their atoms are neatly arranged. These components can typically be studied with conventional X-ray diffraction (XRD). But battery interphases also contain unorganized, amorphous components whose characterizations are beyond the capabilities of XRD. Instead, a technique called pair distribution function (PDF) analysis is needed.
At the XPD beamline, led by Sanjit Ghose, scientists can conduct both techniques simultaneously. With these two techniques, the researchers can understand all the chemical species that evolve during the reactions that form the interphase components.
“We call this combined method total scattering,” explained Ghose, who is a co-author on the paper. “But these techniques are especially unique because they can characterize the structures of chemical species reliably—even if they are only present in trace amounts—which is needed for battery research.”
“Enyuan’s group is really becoming a champion of leveraging XPD’s total scattering techniques and its ability to not damage samples,” he added.
The scientists found that the cesium nitrate additive increased the presence of components known to make the interphase more protective. The XRD data, however, had a surprise in store. In addition to the typical crystalline components, a compound called cesium bis(fluorosulfonyl)imide was also identified.
“This component of the interphase had never been reported before,” said Rahman, emphasizing the novelty of the finding.
“But it’s not just about what we found,” added Hu. “It’s also what was missing from the interphase.”
Scientists studying batteries generally regard lithium fluoride as a necessary component of a good interphase. In fact, its presence and abundance are typically used to explain the impressive performance of lithium metal batteries. That’s why Rahman and Hu were especially surprised by its absence.
“We don’t know why it is not there,” Hu said. “But the fact that this lithium fluoride-free interphase enables a long cycle life and fast charging inspires us to revisit the current understanding of the interphase.”
Though the XPD beamline is adept at detecting trace amounts of interphase components, it is difficult to use the same X-ray beams to quantify these components—especially when some of them are present in such small amounts. So, the scientists brought their batteries to the Submicron Resolution X-ray Spectroscopy (SRX) beamline to quantitatively analyze how the different chemical elements collected on the battery electrodes and in their respective interphases after cycling.
To do this, the SRX beamline scientists used an ultra-sensitive technique called scanning X-ray fluorescence (XRF) microscopy. This technique, which is based on a known and calibrated standard, evaluates the chemical distribution of the interphase.
The scanning XRF images confirmed that there was more cesium present in the anode interphase than the cathode interphase. With further scanning XRF analysis, the scientists revealed that the cesium nitrate additive prevented the breakdown of the transition metals that make up the cathode, contributing to the overall stabilization of the cathode and lithium metal battery.
The scientists also analyzed their samples at the Quick X-ray Absorption and Scattering (QAS) and the In situ and Operando Soft X-ray Spectroscopy (IOS) beamlines to verify that cesium accumulated on the lithium metal anode and nitrate accumulated on the cathode, respectively. Furthermore, the IOS beamline scientists confirmed that the cathode was stabilized with the cesium nitrate additive.
QAS beamline scientists take advantage of the beamline’s high energy X-rays, which can probe deep into the sample, to conduct hard X-ray absorption spectroscopy (XAS). Scientists at the IOS beamline, on the other hand, use low energy X-rays to directly probe atoms near the surface of the sample. Both techniques provide detailed analyses of the chemical and electronic states of the atoms present at the respective electrodes.
“Conducting complementary analyses at these additional beamlines helped us verify our design idea,” said Hu. The two XAS techniques were crucial for characterizing the anode and cathode as well as the interphase.
But the scientists’ analyses were not yet complete; they also had to check for stabilization of the lithium metal anode with the cesium nitrate additive. So, the scientists brought their batteries to the materials synthesis and characterization facility at the Center for Functional Nanomaterials (CFN), a DOE Office of Science user facility at Brookhaven Lab, to make use of the scanning electron microscope.
The resulting microscope images showed that the lithium formed by electrochemical reactions deposits uniformly when the cesium nitrate is added to the electrolyte, thus contributing to the stabilization of the electrode and reinforcing the benefits of this additive.
“We really took advantage of all the resources available to us at Brookhaven Lab,” said Rahman.
By combining various techniques across two user facilities, the scientists were able to paint a full picture of how the lithium metal battery behaves with the cesium nitrate additive. This research contributes to a better understanding of interphase optimization and overall battery chemistry.
“Lithium metal batteries have come a long way, but they still have a long way to go. The interphase plays a key role in progress that still needs to be made,” Rahman said. “Our work has created new opportunities for interphase engineering, and I hope that this will inspire others to look at the interphase differently so that we can accelerate the development of lithium metal batteries.”
More information:
Muhammad Mominur Rahman et al, An inorganic-rich but LiF-free interphase for fast charging and long cycle life lithium metal batteries, Nature Communications (2023). DOI: 10.1038/s41467-023-44282-z
Provided by
Brookhaven National Laboratory
Citation:
Electrolyte additive increases charging rate of lithium metal batteries (2024, January 26)