An unexpected observation has led researchers at the Weizmann Institute of Science to challenge a 200-year-old doctrine regarding the embryonic origins of the pituitary gland.
Situated at the base of the brain, this pea-sized organ, also known as the hypophysis, plays a central role in maintaining body metabolism. Interfacing between the brain and the blood, it can be described as the control center of the endocrine system, which releases hormones into the bloodstream.
The findings of the new study, conducted in Prof. Gil Levkowitz’s lab in Weizmann’s Molecular Cell Biology and Molecular Neuroscience Departments, may improve our understanding of the interplay between different cells in the pituitary and shed new light on the gland’s disorders. The research is published in the journal Developmental Cell.
The structure of the pituitary, which contains two separate lobes that serve different physiological functions, has been highly conserved throughout evolution, meaning that fish, mouse and human pituitary glands are largely similar. For many years researchers took great interest in a fundamental question: Where do the two lobes originate during embryonic development?
The early embryo consists of three primary cell layers, from which the entire body ultimately arises: The endoderm (the inner layer), the mesoderm (middle layer) and the ectoderm (outer layer). Until now, the generally accepted view was that the cells making up each of the two lobes of the pituitary originated from separate embryonic subdivisions of the ectoderm.
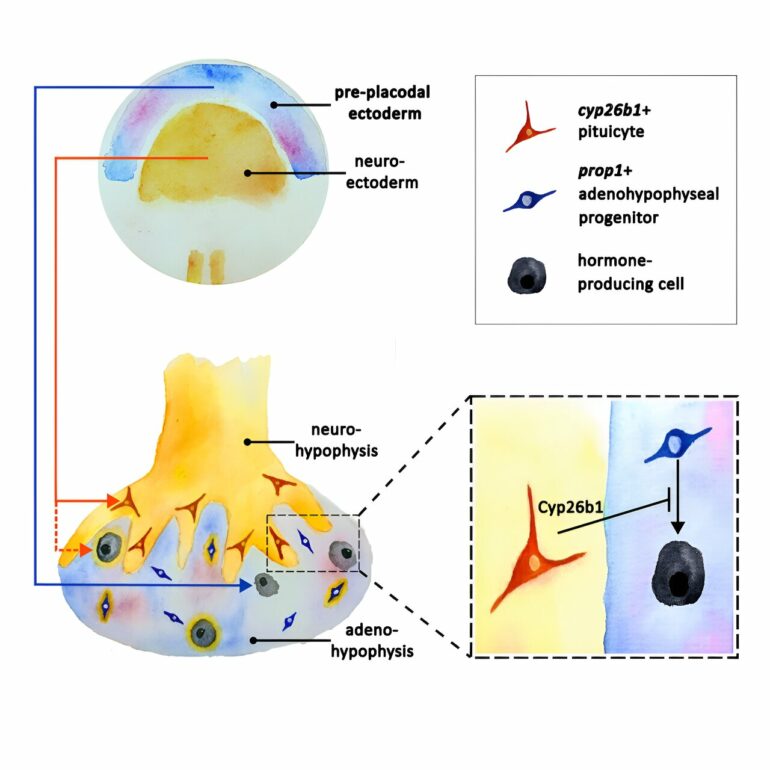
The frontal, or anterior lobe, which releases six major hormones—including the thyroid-stimulating and growth hormones—was thought to originate solely from the early embryo’s exterior tissue layer, the oral ectoderm. The posterior lobe, which releases two major brain-derived hormones—oxytocin, a regulator of reproduction and behavior, and vasopressin, which controls various aspects of body fluid balance—was thought to originate from the neural ectoderm, a tissue that eventually also forms the nervous system. This subdivision was first introduced in 1838 by Martin H. Rathke, a pioneering German embryologist, and it received support from subsequent studies.
But when Ph.D. student Qiyu Chen decided to revisit Rathke’s theory on the pituitary’s origin, she noticed something contrary to expectation. Taking advantage of new genetic and imaging methodologies unavailable to earlier researchers, Chen was able to tag various cells in the ectodermal tissue of a zebrafish embryo and visually trace what happened to progenies of these cells as the embryo assumed its mature shape and the pituitary formed.
In line with the prevalent dogma, she expected the frontal lobe of the fish’s pituitary to contain only cells with genetic labels from the early embryo’s oral ectoderm, and the posterior lobe, from the embryo’s neural ectoderm. Instead, she found that some of the cells in the frontal lobe were descendants of the embryo’s neural ectoderm.
“This finding contradicted the idea that the two parts of the pituitary gland have entirely separate origins,” Levkowitz says. “There had been hints in research by other scientists that these origins might be mixed, but before our study, no one had produced the smoking gun.”
The smoking gun supplied by Levkowitz’s team consisted of cells belonging to a specific type that lit up in the “wrong” pituitary lobe. The researchers were able to reveal them by combining Chen’s genetic tracing studies in the developing embryo with single-cell transcriptomics, an advanced technique that they applied in collaboration with the lab of Prof. Ido Amit of Weizmann’s Systems Immunology Department. The scientists were thus able to unravel the precise molecular composition of individual cells and follow their development.
They then learned that a similar, unpublished finding concerning the mixed origins of pituitary lobe cells had been made in mice at the Francis Crick Institute in London. The lead researcher of that study, Dr. Karine Rizzoti, was labeling cells in the neural ectoderm of mice embryos. When she later found that the progenies of some of those cells were detected in the frontal lobe of the mouse pituitary, she was initially skeptical of her results.
“We shared with her that we had made similar observations using fish,” says Levkowitz, who invited Rizzoti to collaborate with his team on this project. “We decided to join forces to make a stronger case, with two species,” he explains.
By identifying the exact molecular signatures of the major cell types in the pituitary, the project also led to an additional finding: Previously unknown cross-talk between different cells belonging to the frontal and posterior parts of the gland.
The researchers discovered that certain cells in the posterior lobe, called pituicytes, influence the development of hormone-producing cells in the frontal lobe. The pituicytes, a subtype of the astroglia—star-shaped cells of the nervous system—were known to facilitate the release of oxytocin and vasopressin hormones from the posterior pituitary lobe.
“Our finding was a surprise—in addition to their previously known function, pituicytes play a role in the development of the frontal pituitary,” says Chen.
“We know a great deal about the anatomy of the pituitary gland, but there is still much to be learned about its genetic composition,” Levkowitz says. “Understanding this composition in different cells, how it comes about in early embryonic development and how the different cell types affect one another, may help us figure out what goes wrong in various diseases involving the pituitary.
“These include cancer and certain childhood diseases, such as congenital growth hormone deficiency. In fact, the latter deficiency occurs because of mutations in a gene that affects the decision of early embryonic ectodermal cells to become hormone-producing cells of the frontal pituitary lobe.”
Levkowitz points out that for the most part, the dogma regarding the separate origins of the pituitary’s two lobes is still correct. “However, our discovery that a small proportion of hormone-producing cells in the frontal lobe originate from a different part of the embryonic tissue than was previously thought, might open up new ways of exploring malfunctions of the pituitary.”
Explains Chen, “For example, let’s say you have 100 growth hormone cells. Ninety-five come from the oral ectoderm, as was commonly believed, but now it turns out, surprisingly, that five of them have a neural origin. All 100 cells release the same hormone, but perhaps they do so in response to different physiological demands.
“Learning the exact nature of these signals might in the future lead to improved ways of correcting hormonal deficiency—by targeting specific pituitary cells while avoiding unwanted effects on vital pituitary endocrine functions.”
More information:
Qiyu Chen et al, Neural plate progenitors give rise to both anterior and posterior pituitary cells, Developmental Cell (2023). DOI: 10.1016/j.devcel.2023.08.018
Provided by
Weizmann Institute of Science
Citation:
Pituitary gland’s embryonic origins may lead to new insights on growth hormone deficiency (2024, February 2)