When designing repositories for high-level radioactive waste in deep geological layers, various factors must be carefully considered to ensure their long-term safety. Among other things, natural communities of microorganisms can influence the behavior of the waste, especially when it comes into contact with water. The microorganisms interact with released radionuclides and influence their mobility.
Researchers at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) have taken a closer look at a microorganism that occurs in the vicinity of a potential repository. Their findings are published in the journal Science of The Total Environment.
In Germany, rocks that are suitable for the permanently safe storage of highly radioactive waste in a repository—so-called host rocks—are certain clay rock formations in addition to rock salt and crystalline rock. A multi-barrier system is preferred, consisting of the waste container as a technical barrier, the backfill material as a geotechnical barrier and the host rock as a geological barrier. This system is intended to isolate the radioactive waste from the environment.
“The combination of clay formations with the backfill material bentonite, which consists of various clay minerals, is an example of such a system. We know that so-called sulfate-reducing microorganisms occur in both the host rock and in the backfill material. In our work, we investigated a representative of the genus Desulfosporosinus in more detail. We were particularly interested in its influence on uranium present in the bentonite-clay system,” explains Dr. Stephan Hilpmann from the HZDR Institute of Resource Ecology.
Uranium can occur in a variety of compounds and can assume different oxidation states. In natural deposits, uranium is mainly found in tetravalent and hexavalent form. Under normal conditions, tetravalent uranium compounds—in contrast to hexavalent compounds—are almost insoluble in water. Uranium compounds are toxic, whereby the toxicity depends mainly on their solubility. This distinct behavior of the compounds with different oxidation states is of great importance for understanding the processes in the repository.
Microbial defense removes uranium from water
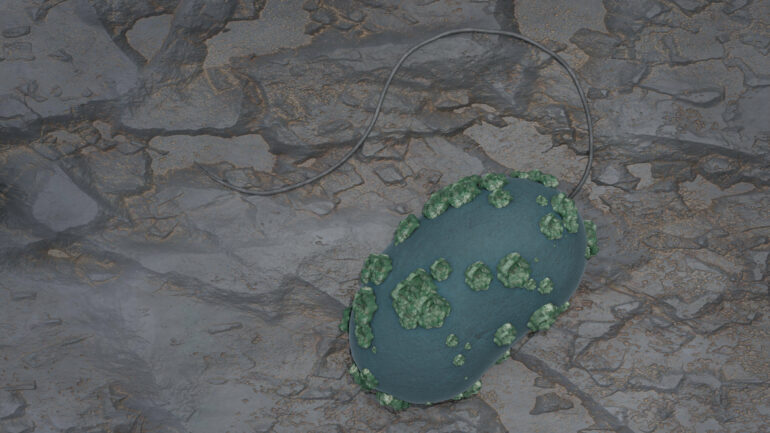
Desulfosporosinus lives under anaerobic conditions: It only grows in the absence of air. This allowed the researchers to study the microorganism under realistic conditions, such as those found in deep layers of rock. To do this, they brought the bacterial cultures into contact with uranium salt solutions in natural pore water of the clay rock, covered by a nitrogen atmosphere that protects them from atmospheric oxygen.
They observed that the bacteria convert the easily water-soluble hexavalent uranium into sparingly soluble tetravalent uranium. The bacteria can deposit this sparingly soluble uranium in membrane vesicles on their cell surface in the form of incrustations.
The team assumes that this is a defensive reaction of the microorganisms—a behavior that has previously been observed in other types of bacteria.
“After one week, the bacteria have converted about 40 percent of the originally dissolved uranium into the poorly soluble variant,” Hilpmann reports.
The team also observed a further oxidation stage with pentavalent uranium, about whose formation in this process not much was previously known. This is mainly due to its typical instability. The researchers suspect that they were only able to detect pentavalent uranium because the bacteria stabilize it to some extent in solution. They were able to detect this oxidation state even after one week.
Multispectral view into contaminated subsurface
To observe the various uranium compounds, the team used a range of modern spectroscopy and microscopy methods. The HZDR researchers have access to highly specialized techniques at the Institute of Ion Beam Physics and Materials Research and at the Rossendorf Beamline (ROBL), which the HZDR operates at the European Synchrotron Radiation Facility (ESRF) in Grenoble. At the French site, for example, they can investigate radiochemical processes spectroscopically. Here they have also observed the formation of pentavalent uranium in the process using a method called HERFD-XANES.
HERFD-XANES stands for fluorescence detection with high-energy resolution, which is coupled with X-ray near-edge absorption spectroscopy. This is an X-ray absorption spectroscopic method that can be used to study the behavior of electrons. The team was able to visualize the uranium-containing aggregates on the cell surface of Desulfosporosinus using scanning transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy.
“Our findings deepen our understanding of the complex processes in a potential final repository. They may also be relevant for the removal of radioactive pollutants from contaminated waters and thus for their remediation,” says Hilpmann.
More information:
Stephan Hilpmann et al, Presence of uranium(V) during uranium(VI) reduction by Desulfosporosinus hippei DSM 8344T, Science of The Total Environment (2023). DOI: 10.1016/j.scitotenv.2023.162593
Provided by
Helmholtz Association of German Research Centres
Citation:
Uranium-immobilizing bacteria in clay rock: Exploring how microorganisms can influence the behavior of radioactive waste (2024, April 16)