Hydrogen is often seen as the fuel of the future on account of its zero-emission and high gravimetric energy density, meaning it stores more energy per unit of mass compared to gasoline. Its low volumetric density, however, means it takes up a large amount of space, posing challenges for efficient storage and transport.
In order to address these deficiencies, hydrogen must be compressed in tanks to 700-bar pressure, which is extremely high. This situation not only incurs high costs but also raises safety concerns.
For hydrogen-powered fuel-cell vehicles (FCVs) to become widespread, the US Department of Energy (DOE) has set specific targets for hydrogen storage systems: 6.5% of the storage material’s weight should be hydrogen (gravimetric storage capacity of 6.5 wt%), and one liter of storage material should hold 50 grams of hydrogen (a volumetric storage capacity of 50 g L‒1). These targets ensure that vehicles can travel reasonable distances without excessive fuel.
One promising strategy to achieve these targets is to develop porous adsorbent materials, such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and porous organic polymers (POPs). All these materials share a common feature: they possess a porous structure that allows them to effectively trap and store hydrogen gas. This approach also aims to facilitate hydrogen storage at lower pressure, such as within 100 bar.
Despite advancements in surpassing the DOE’s gravimetric target, many adsorbent materials still struggle to meet volumetric capacity needs, and few can balance both volumetric and gravimetric targets. From an industrial standpoint, volumetric capacity is more crucial than gravimetric capacity, as vehicle storage tanks have limited space.
A hydrogen storage system’s volume directly impacts the driving range of FCVs. Therefore, developing hydrogen adsorbents that maximize volumetric capacity while maintaining excellent gravimetric capacity is essential. Achieving this goal involves balancing a high volumetric and gravimetric surface area within the same material.
Researchers are investigating various materials for hydrogen storage, with organic supramolecular crystals assembly from organic molecules through noncovalent interactions, being a promising option as a result of their recyclability. Their potential remains largely untapped, however, because designing supramolecular crystals with balanced high gravimetric and volumetric surface areas, while maintaining stability, is difficult.
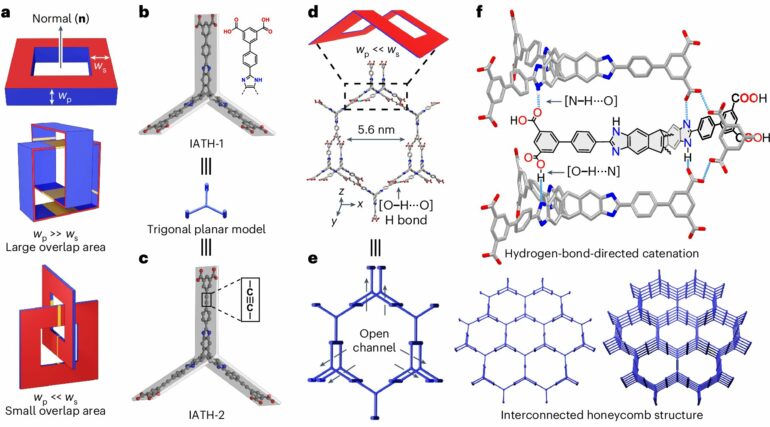
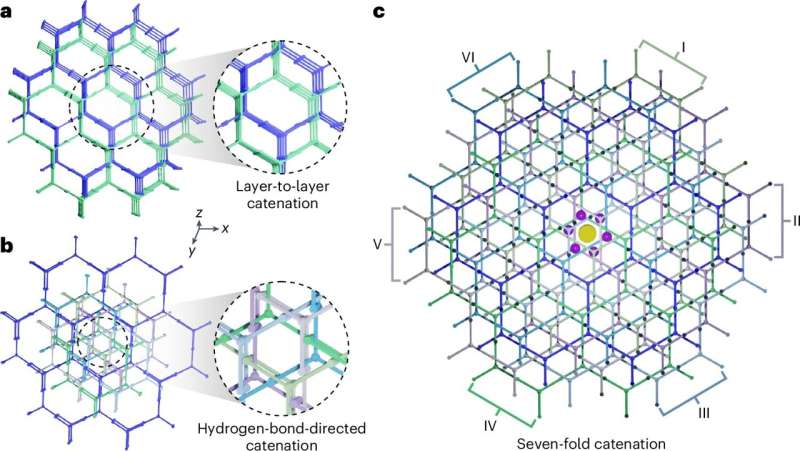
A phenomenon known as catenation, which involves mechanically interlocked networks in porous materials, typically enhances stability. Catenation, however, often reduces surface area by blocking accessible surfaces, making the material less porous and generally undesirable for hydrogen storage. Efforts are usually made to minimize or avoid it.
Interpenetration analysis of RP-H100 and RP-H101. © Nature Chemistry (2024). DOI: 10.1038/s41557-024-01622-w
To unlock the potential of supramolecular crystals for hydrogen storage, a collaborative research team led by Professor Fraser STODDART, along with Research Assistant Professors, Dr. Chun Tang, Dr. Ruihua Zhang from the Department of Chemistry, The University of Hong Kong (HKU), and Professor Randall Snurr from the Department of Chemical and Biological Engineering, Northwestern University, US, demonstrated a controlled “point-contact catenation strategy.”
The research is published in the journal Nature Chemistry.
This innovative approach uses hydrogen bonds, the cross-section of which can be seen as a “point,” rather than the traditional [π···π] stacking which involves large “surface” overlap, to guide catenation in a precise manner in supramolecular crystals. Based on this strategy, researchers create a well-organized framework that minimizes surface loss caused by interpenetration and tailors the pore diameter (~1.2–1.9 nm) for optimal hydrogen storage.
As a result, the research team obtained a supramolecular crystal with record-high gravimetric (3,526 m2 g‒1) and balanced volumetric (1,855 m2 cm‒3) surface areas among all the reported (supra)molecular crystals, in addition to high stability, while (i) bringing about excellent material-level volumetric capacity (53.7 g L‒1), (ii) balancing high gravimetric capacity (9.3 wt%) for hydrogen storage under practical pressure and temperature swing conditions (77 K/100 bar → 160 K/5 bar), and (iii) surpassing the DOE ultimate system-level targets (50 g L‒1 and 6.5 wt%) both volumetrically and gravimetrically, albeit at cryogenic temperatures.
Innovative design
Designing organic supramolecular crystals that balance high gravimetric and volumetric surface areas, while also maintaining high stability, is a momentous challenge, which has hindered its potential for many applications.
The team, however, has proposed a point-contact catenation strategy that utilizes point-contact interactions involving hydrogen bonding to minimize surface loss during catenation. This design strategy endows these supramolecular crystals with balanced high volumetric and gravimetric surface areas, high stability, and ideal pore sizes for hydrogen storage.
This research unlocks the potential of organic supramolecular crystals as promising candidates for onboard hydrogen storage and highlights the potential of a directional catenation strategy in designing robust porous materials for applications.
More information:
Ruihua Zhang et al, Balancing volumetric and gravimetric capacity for hydrogen in supramolecular crystals, Nature Chemistry (2024). DOI: 10.1038/s41557-024-01622-w
Provided by
The University of Hong Kong
Citation:
Organic supramolecular crystals with high hydrogen storage performance could enhance fuel-cell vehicle efficiency (2024, September 27)