Implantation is the initial step in pregnancy, where the embryo attaches to and enters the endometrium, the inner tissue layer of the uterus. During this process, the cells of the endometrium change to build the right conditions for the fertilized egg to develop.
Publishing in Communications Biology, a research team led by Professor Kei Miyamoto from Kyushu University’s Faculty of Agriculture, along with Dr. Isao Tamura and Professor Norihito Sugino of Yamaguchi University, have elucidated a key step in how the cells of the endometrium change. When endometrial stroma cells, or ESCs, differentiate into cells suitable for egg implantation, the actin in the cell’s nucleus dynamically changes, flipping the switch for the cell’s differentiation process.
In the initial steps of human pregnancy, the uterus undergoes many changes to build a suitable environment for an embryo to implant and grow. One of these changes is called decidualization. This is when ESCs alter their function and morphology, and differentiate into decidual cells that are critical for implantation and maintenance of pregnancy.
“If decidualization is impaired the embryo cannot be implanted, leading to infertility. However, the regulatory mechanism of decidualization has not yet been completely elucidated,” explains Miyamoto. “Tamura and Sugino’s group previously reported that during decidualization, the expression of many genes change. To gain more molecular insight into this process, we focused on actin proteins.”
Actin is a component of the cell’s structure, or cytoskeleton, and is known to be involved in changing a cell’s shape, a key step in decidualization. Recently, actin has been found to exist in the nucleus of ESCs.
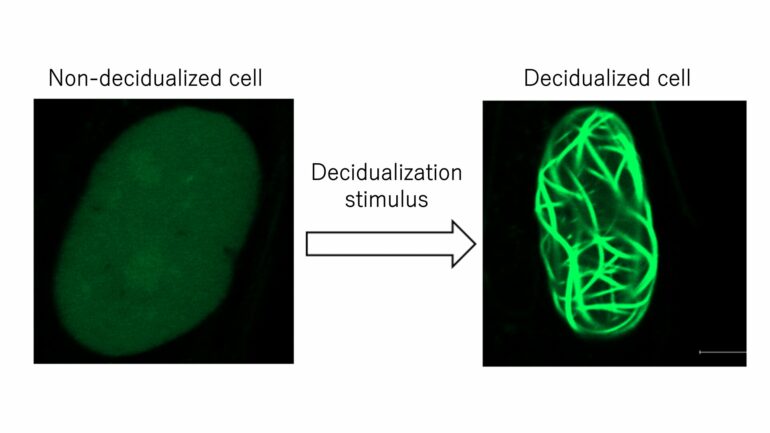
The team began by developing ESCs where they could visually track the behavior of nuclear actin in real time. In their analysis, they found that once decidualization began, actin in the nucleus aggregates into fibers.
“Interestingly, when the cells are returned to their original ESC state, the nuclear actin aggregates also disappeared. Artificially inhibiting the formation of actin aggregates inhibited decidualization, indicating that its formation is essential for the process,” continues Miyamoto. “Further analysis revealed that this assembly of actin played a role in suppressing cell proliferation.”
During decidualization, cell proliferation needs to temporarily stop for the ESCs to begin their differentiation process. The team was successful in identifying a transcription factor named C/EBPb as the key factor that controls the formation of nuclear actin assembly during this entire process.
This discovery elucidates a new mechanism of nuclear actin-mediated control in the decidualization process and reveals a new role for nuclear actin in the implantation process.
“It is worth considering targeting nuclear actin dynamics and its regulatory factor C/EBPb for developing new treatments against implantation failure,” concludes Miyamoto.
More information:
Isao Tamura et al, Nuclear actin assembly is an integral part of decidualization in human endometrial stromal cells, Communications Biology (2024). DOI: 10.1038/s42003-024-06492-z
Citation:
Uncovering new regulatory mechanisms in embryo implantation (2024, October 18)