Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences have recently developed a neotype lysosomal trap for clearing viruses and variants. This lysosomal “TRAP” (lysoTRAP) shows efficient viruses and variants infection inhibition potential in cell, mouse, hamster, and human lung organoid models.
Results of the study are published in Nature Communications.
Viral infections pose a serious threat to human health and safety. The viral donors specifically binding to the receptors of host cells is essential for virus entry. Multiple studies have explored the development of medical agents (such as antibodies, small molecule inhibitors) to prevent this binding.
The effectiveness of these agents (and virtually all vaccines) relies on their specificity to the viral donors; therefore, these strategies can be rendered ineffective by the virus mutation, which is now known to occur frequently and poses a continuous threat to public health.
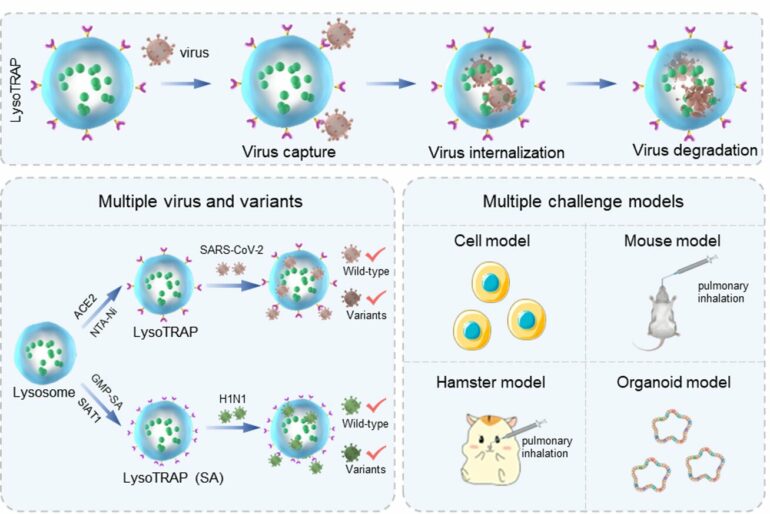
Inspired by the idea of mimicking virus entry to host cells and macrophage-mediated virus clearance and taking severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a testbed, the IPE researchers initially modified the surface of lysosomes with the viral protein-receptor angiotensin-converting enzyme 2 (ACE2) as bait, thus constructing a lysoTRAP that could selectively capture, internalize, and degrade SARS-CoV-2.
“This designed lysoTRAP is a pioneer to explore the lysosome as a therapeutic, and represents a novel anti-virus strategy,” said Prof. Ma Guanghui from IPE.
It initially exploited the spike/ACE2 affinity to capture SARS-CoV-2, internalized the virus to completely isolate them from the host cells, and then degraded this virus by the lysosomal hydrolases to profoundly reduce the likelihood of viral escape or continued infectivity, according to Prof. Ma.
In the cell model, lysoTRAP efficiently inhibited the infection of wild-type pseudotyped SARS-CoV-2 and nine variants. In the hamster model, it cleared both authentic wild-type SARS-CoV-2 and the omicron variant, thus alleviating viral pneumonia.
To approach the genetic and epigenetic features of human lung cells, researchers established a human lung organoid model and found that lysoTRAP outperformed the current standard of care (EIDD-2801 [riboside analogs] and RBD-antibody) against wild-typed SARS-CoV-2. Working with the omicron variant, the inhibitory effect of either free EIDD-2801 or RBD antibody showed an obvious decrease, but lysoTRAP did not.
Distinct from other therapeutic agents that always targeted the viral donors, lysoTRAP was not vulnerable to loss of efficacy from virus mutations. “Given the working principle for lysoTRAP’s recognition of SARS-CoV-2 is the ACE2 protein, any infective variants that bind ACE2 are sensitive to lysoTRAP-mediated clearance,” said Prof. Wei Wei from IPE.
Extending beyond SARS-CoV-2, researchers replaced protein-receptor ACE2 with saccharide-receptor sialic acid (SA) and again found potent clearance of influenza A by the lysosomal trap in cell, mouse, and human lung organoid models, demonstrating the flexibility of the lysoTRAP system.
The proposed lysoTRAP platform “holds significant promise for therapeutic development, offering a unique strategy to clear viral infections by leveraging the natural degradative functions of lysosomes,” and introduced “a very innovative approach for combating viral infections,” according to a peer reviewer from Nature Communications.
More information:
Chengliang Lyu et al, Lysosomal “TRAP”: a neotype modality for clearance of viruses and variants, Nature Communications (2024). DOI: 10.1038/s41467-024-54505-6
Provided by
Chinese Academy of Sciences
Citation:
Researchers develop lysosomal ‘TRAP’ for clearance of viruses and variants (2024, November 26)