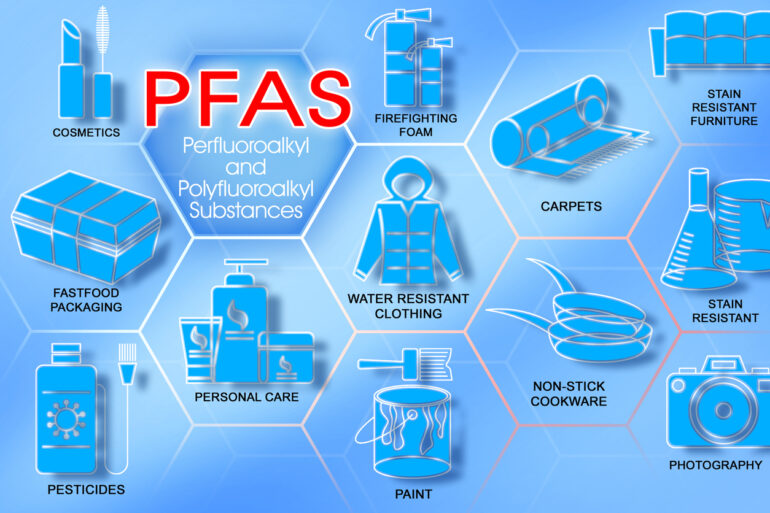
Perfluoroalkyl and polyfluoroalkyl substances, or PFAS, have earned the nickname of forever chemicals from their extraordinary ability to stick around in the environment long after they’ve been used.
These synthetic compounds, commonly used in consumer products and industrial applications for their water- and grease-resistant properties, are now found practically everywhere in the environment.
While many chemicals will degrade relatively quickly after they’re disposed of, PFAS can stick around for up to 1,000 years. This durability is great for their use in firefighting foams, nonstick cookware, waterproof clothing and even food packaging.
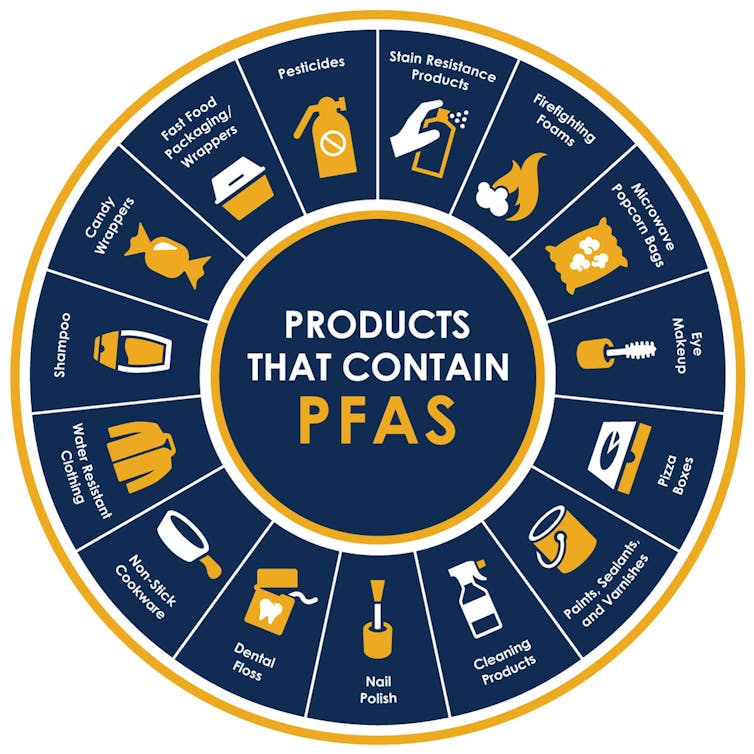
These are a few examples of products that can contain PFAS.
City of Riverside, California
However, their resilience means that they persist in soil, water and even living organisms. They can accumulate over time and affect the health of both ecosystems and humans.
Some initial research has shown potential links between PFAS exposure and various health issues — including cancers, immune system suppression and hormone disruption. These concerns have led scientists to search for effective ways to break down these stubborn chemicals.
We’re a team of researchers who developed a chemical system that uses light to break down bonds between carbon and fluorine atoms. These strong chemical bonds help PFAS resist degradation. We published this work in Nature in November 2024, and we hope this technique could help address the widespread contamination these substances cause.
Why PFAS compounds are so hard to break down
PFAS compounds have carbon-fluorine bonds, one of the strongest in chemistry. These bonds make PFAS incredibly stable. They resist the degradation processes that usually break down industrial chemicals – including hydrolysis, oxidation and microbial breakdown.
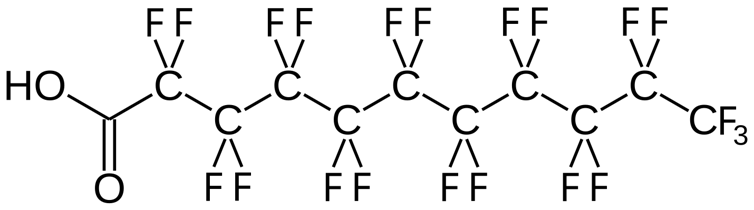
The carbon-fluorine bonds in PFAS, like this one, perfluoroundecanoic acid, make the molecules very stable.
Bert.Kilanowski/Wikimedia Commons
Conventional water treatment methods can remove PFAS from water, but these processes merely concentrate the contaminants instead of destroying them. The resulting PFAS-laden materials are typically sent to landfills. Once disposed of, they can still leach back into the environment.
The current methods for breaking carbon-fluorine bonds depend on use of metals and very high temperatures. For example, platinum metal can be used for this purpose. This dependence makes these methods expensive, energy-intensive and challenging to use on a large scale.
How our new photocatalytic system works
The new method our team has developed uses a purely organic photocatalyst. A photocatalyst is a substance that speeds up a chemical reaction using light, without being consumed in the process. Our system harnesses energy from cheap blue LEDs to drive a set of chemical reactions.
After absorbing light, the photocatalyst…