
Curious Kids is a series for children of all ages. If you have a question you’d like an expert to answer, send it to [email protected].
What is rust? – Henry E., age 13, Boston, Massachusetts
Imagine leaving your shiny metal bicycle outside in the rain. As water pools on its surfaces, oxygen from the air lingers nearby, and together they begin to quietly attack the metal.
The iron in the bike and the oxygen and water in the environment together undergo a chemical reaction. It forms iron oxide – better known as rust – which accumulates over time. This reddish-brown, flaky substance is more than just ugly; it’s a sign that the metal is breaking down.
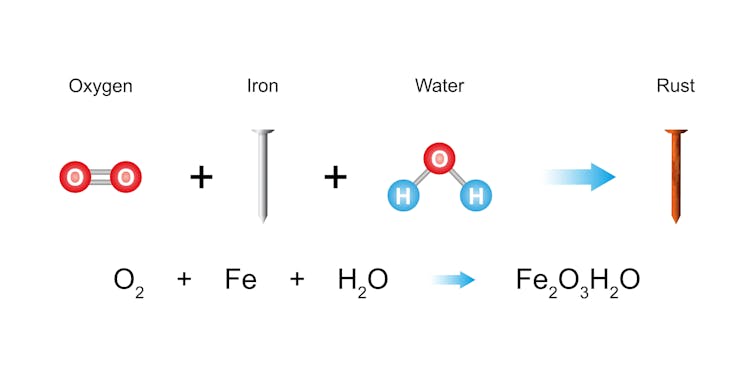
Iron reacts with oxygen and water to form rust, an oxidized form of iron.
Ali Damouh/Science Photo Library via Getty Images
Chemists call this process oxidation. You can think of iron as like a superhero — tough, durable and shiny — until it meets its kryptonite: moisture and air. Water helps iron atoms more easily shed their electrons, which are negatively charged particles. Oxygen acts like a tiny electron thief, stealing those electrons and leaving the metal weak and crumbly.
The shiny, metallic iron used in homes and industry is a refined form of what is found in nature — iron ore. Rust is a natural process — the refined iron is essentially trying to return to its original oxidized, stable state: iron ore.

Old water pipes can clog with accumulated rust.
chimmy/E+ via Getty Images
From bikes and cars to bridges and ships
From household fixtures to monumental machines, rust moves in wherever metal meets moisture and time.
On bikes and cars, the combination of rain and exposed metal often triggers a full-blown rust party, eating away at frames and undercarriages. Old water pipes are another hot spot — over time, they corrode from the inside, often leaking brown, rusty water into sinks and tubs. In the kitchen, standing water left around sinks or faucets can lead to yellow-orange rust stains that are as stubborn to remove as they are unsightly.
On a much larger scale, rust can wreak havoc on ships and bridges. Corroded hulls can lead to oil leaks or even catastrophic sinking, costing the maritime industry billions of dollars each year in repairs and environmental damage.
And here’s a twist – salt speeds up the rusting process. In snowy regions, road salt doesn’t just melt ice; it also turbocharges oxidation, accelerating the corrosion process. That’s why cars in snowy places might rust faster.

The copper Statue of Liberty is greenish due to oxidization, which forms the colorful patina, the copper version of rust.
ErickN/iStock via Getty Images Plus
Rust is the term for the specific type of corrosion that occurs in iron or steel. But any metal can chemically degrade due to reactions…



