One of the most challenging aspects of cancer treatment is the huge variety of different tumors that can occur with each one potentially requiring a different solution because unfortunately, one drug does not fit all. In addition, another major issue of many current drugs is their poor selectivity towards cancers resulting in problems such as normal tissue toxicity, severe side effects and the development of drug resistance.
Now, a team of scientists at the University of Huddersfield is researching how to combat these challenges by using “self-assembled” drugs, and although the research is in its very early stages, they’ve already had a breakthrough.
The science behind self-assembly
Self-assembly is the ability to instruct chemical systems with specific information so in the correct environment they will spontaneously generate biologically active compounds. Using this process many different compounds can be rapidly and easily formed with each different self-assembled drug having different chemotherapy properties.
“In the future, it may be possible to target many cancers through this approach with the correct drug ‘self-assembled’ in such a manner to be selective for a specific cancer,” says Professor Craig Rice.
In an article published by the journal Nature Communications, the University’s Professor Roger Phillips, Dr. Simon Allison and Professor Craig Rice demonstrate chemical systems that self-assemble into molecular capsules which are highly toxic towards human cancer cells of a range of different tumor types.
More importantly, they show unprecedented cancer selectivity in the laboratory, that in some cases, is many thousands of times more toxic to the cancer cells compared to healthy, normal cells.
Cancer therapy without severe side effects
Eventually, if similar results are obtained in more complex testing systems including in patients, and once considered safe after careful testing, it could offer the possibility of being able to treat cancers without the severe side effects commonly associated with chemotherapy drugs.
“Anti-cancer drug discovery and development can be enormously time consuming and expensive with a particular drug only being effective against a relatively small number of cancers with specific shared properties,” explained Professor Rice, who heads the University’s Department of Chemical Sciences and is also the Director of the Structural, Molecular and Dynamic Modelling Centre within the School of Applied Sciences.
“In the future, it may be possible to target many cancers through this approach with the correct drug ‘self-assembled’ in such a manner to be selective for a specific cancer,” he said.
Targeting hard-to-treat cancers
The research could also pave the way in targeting hard-to-treat cancers for which commonly used chemotherapy drugs have little or no effect.
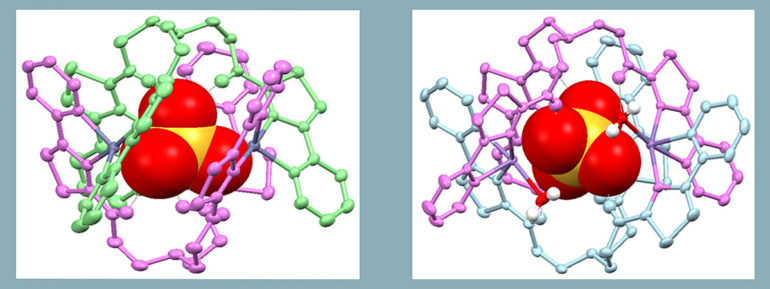
The published studies show the new potential drug can be assembled with either zinc, copper, or manganese, with the three metal ions imparting significantly different chemotherapeutic properties via different mechanisms dependent upon which metal ion is used.
“It is this which allows the generation of different chemical systems each of which may have specificity for different cancers,” he said.
Two different compounds were generated (seen pictured in the attached diagram) using zinc (left) and manganese (right). Whilst these two compounds “look” very similar, the zinc compound demonstrated excellent anti-cancer activity and selectivity towards a range of cancers in the laboratory, whereas the manganese compound was comparatively much more toxic, meaning there was more anti-cancer activity at a lower concentration, with similar selectivity compared to current drugs tested.
Future studies will test whether this may be useful for cancers for which effective treatments are not currently available.
Professor Rice added that these studies represent the very early days of drug discovery, and whilst the initial results have been very promising, there will be many obstacles to overcome before the full clinical potential of this new discovery is realized.
Bio-orthogonally catalyzed lethality strategy generates targeting drugs from within tumors
More information:
Simon J. Allison et al, Self-assembly of an anion receptor with metal-dependent kinase inhibition and potent in vitro anti-cancer properties, Nature Communications (2021). DOI: 10.1038/s41467-021-23983-3
Provided by
University of Huddersfield
Citation:
Team achieves cancer therapy breakthrough in vitro using ‘self-assembled’ drugs (2021, August 17)
retrieved 18 August 2021
from https://medicalxpress.com/news/2021-08-team-cancer-therapy-breakthrough-vitro.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.