Cholecystokinin (CCK) and gastrin are the earliest discovered gastrointestinal hormones. They are the most abundant peptides in gastrointestinal tract and central nervous system, acting as physiologically important hormones and neurotransmitters through two CCK receptor subtypes, CCKAR and CCKBR.
These two receptors engage in fundamental physiological actions such as satiety regulation, pancreatic enzyme secretion, and gall bladder contraction. They are also implicated in behavioral processes, including anxiety, memory, and drug addiction. However, the development of drugs against cholecystokinin receptors (CCKRs) is challenging mainly due to the lack of precise structural information.
In two studies both published in Nature Chemical Biology, the research team led by Jiang Yi, Wang Mingwei, H. Eric Xu, Zhao Qiang, and Wu Beili from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences and the research team led by Zhao Suwen from ShanghaiTech University, together revealed the mechanisms of ligand recognition, activation and G protein coupling specificity of CCKRs.
The researchers first solved three crystal structures of the human CCKAR in complex with two small-molecule antagonists (lintitript and devazepide) and one full agonist NN9056, as well as five cryo-electron microscopy (cryo-EM) structures of CCK-8 activated CCKAR in complex with three G proteins (Gi, Gs, and Gq) and gastrin activated CCKBR coupled to two G proteins (Gi and Gq). They elucidated the mechanisms of CCKRs recognization by ligands, CCKRs activation, and G protein promiscuity of CCKAR, all of which provide fundamental information for drug discovery of CCKRs.
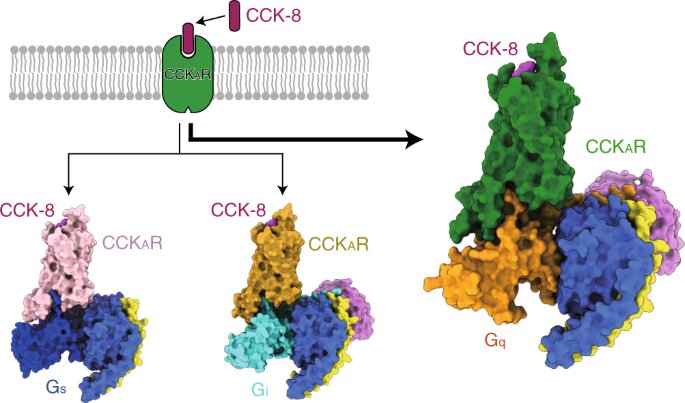
The researchers then presented the structures of sulfated CCK-8 bound CCKAR in complex with Gq, Gs, and Gi heterotrimers at global resolutions of 2.9 angstrom, 3.1 angstrom, and 3.2 angstrom, respectively. They uncovered the binding mode of endogenous peptide CCK-8, and found that the sulfo-tyrosine in CCK-8 was crucial to high affinity of endogenous peptide for CCKAR.
Consistently, the researchers found that Gq protein showed the most potent coupling activity of CCKAR. These results supported Gq as the predominant transducer of CCKAR and highlighted importance of the interface area in G protein coupling selectivity.
Moreover, the researchers reported three crystal structures of CCKAR bound to small molecular antagonists and a peptide agonist, as well as two cryo-EM structures of Gi- and Gq-coupled CCKBR complexes. They revealed the binding mode of CCKRs by both peptide and small molecule ligands, and identified pivotal roles in recognizing CCKAR by devazepide and lintitript, thus providing a template for designing drugs targeting CCKRs.
Combining the inactive and active structures of CCKAR with the molecular simulation analysis, the researchers proposed the stepwise activation process of CCKAR.
The findings of these two studies offered the first insight into ligand recognition and activation of the two CCK receptors and provided a new opportunity for designing drugs targeting CCKRs.
Researchers reveal how lipids and water molecules regulate 5-HT receptors
More information:
Qiufeng Liu et al, Ligand recognition and G-protein coupling selectivity of cholecystokinin A receptor, Nature Chemical Biology (2021). DOI: 10.1038/s41589-021-00841-3
Xuefeng Zhang et al, Structures of the human cholecystokinin receptors bound to agonists and antagonists, Nature Chemical Biology (2021). DOI: 10.1038/s41589-021-00866-8
Provided by
Chinese Academy of Sciences
Citation:
Mechanism of actions of cholecystokinin receptors revealed (2021, October 8)
retrieved 11 October 2021
from https://phys.org/news/2021-10-mechanism-actions-cholecystokinin-receptors-revealed.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.