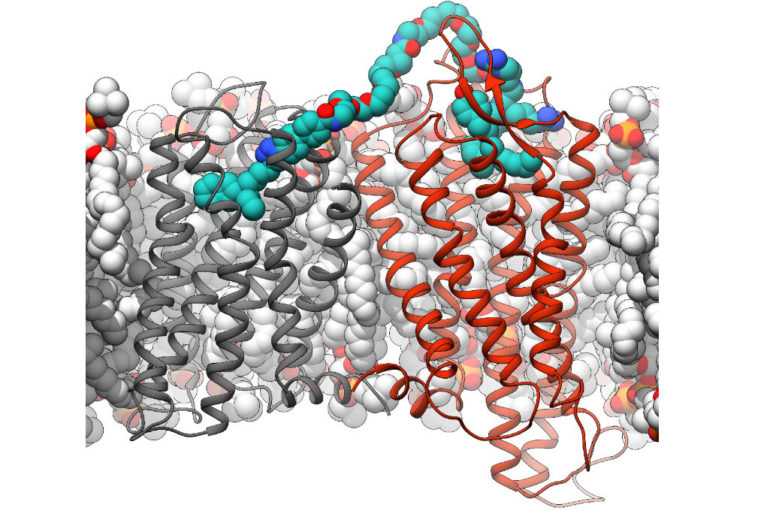
There are a number of G protein-coupled receptors in human cells. As an important component of the cell membrane, these proteins are responsible for detecting different stimuli in the surroundings of a cell within the body and transferring this information to the cell interior. They may act individually or in pairs, and this can have a crucial effect on their function. Together with colleagues from Montreal, Canada, scientists from Friedrich-Alexander Universität (FAU) have investigated G protein-coupled receptors and carried out research into whether tailor-made substances can have an impact on how these receptors form pairs and how they then behave. They have now published their findings in the journal Communications Biology.
G protein-coupled receptors are proteins found in the cell membrane that can detect whether a messenger substance such as a neurotransmitter binds to the cell. This triggers various processes within the cell. Although these receptors are active as individual proteins and are capable of transferring a signal to the cell, researchers also know that different receptors in the cell membrane interact with each other and pairs known as dimers can be formed.
Receptor teams
The team of researchers led by Dr. Dorothée Weikert from the Chair of Pharmaceutical Chemistry at FAU and Professor Michel Bouvier from Université de Montréal have investigated one of these pairs of receptors in more detail. They examined the dimerization of two receptors—one dopamine D3 receptor that detects the neurotransmitter dopamine and one that is activated by neurotensin, a messenger substance that is released by nerve cells. These receptors occur in regions of the brain that play a role in the development of addictions. A team of pharmaceutical chemists at FAU had already developed bivalent ligands that, in contrast to natural neurotransmitters, can bind to both receptors simultaneously.
The team of researchers used these ligands to specifically address the receptor dimers. If both these receptors are bound together using a bivalent ligand and are thereby encouraged to form pairs, the researchers discovered that the way the pair of receptors transmit signals differs from that of single receptors in the cell. If both receptors are connected using the bivalent ligand, the dopamine D3 receptor migrates into the cell interior, something that only rarely occurs with conventional neurotransmitters or substances. This means that the dopamine D3 receptor is only left in small quantities on the surface of the cell.
Future applications
The researchers are currently trying to optimize their bivalent ligands and find out which effects the transfer of the receptor dimer into the cell interior has on the cells themselves. One potential area of application is research into addictions, during which increased levels of the dopamine D3 receptor are present on the cell surface.
Signal transduction without signal-receptor clusters can direct cell movement
More information:
Julian Budzinski et al, Bivalent ligands promote endosomal trafficking of the dopamine D3 receptor-neurotensin receptor 1 heterodimer, Communications Biology (2021). DOI: 10.1038/s42003-021-02574-4
Provided by
Friedrich–Alexander University Erlangen–Nurnberg
Citation:
One plus one does not equal two: Research team investigates receptors that form pairs on the surface of cells (2021, October 25)
retrieved 26 October 2021
from https://phys.org/news/2021-10-equal-team-receptors-pairs-surface.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.