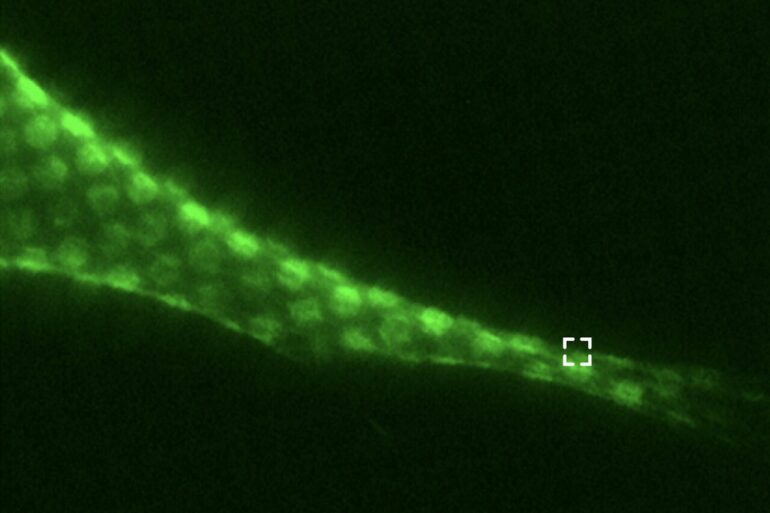
Investigators have discovered that two cytoskeletal proteins which were previously thought to function independently actually interact and form cytoskeletal networks within the cell surface, according to findings published in Proceedings of the National Academy of Sciences (PNAS).
“These findings significantly alter the definition and function of the cell cortex,” said Robert Goldman, Ph.D., professor of cell and developmental biology, of Medicine in the Division of Pulmonary Care and a co-author of the study.
The cytoskeleton of eukaryotic cells is composed of various networks comprising several types of filamentous proteins, including actin and intermediate filaments.
Notably, filamentous actin (F-actin) and vimentin intermediate filaments support cell structure and regulate numerous cell functions; F-actin is found in the cell cortex, closely associated with the surface membrane and regulates the cell’s contractility and movement, while the majority of vimentin intermediate filaments are located within the cell and regulate its shape, structure and mechanical properties during cell movement and migration.
These cytoskeletal proteins have previously been thought to form separate intracellular networks and function independently of one another. However, the current study revealed that wasn’t the case.
Using advanced cell imaging to study cell cultures expressing F-actin and vimentin, the investigators discovered that these cytoskeletal proteins actually work synergistically in the cell cortex.
They found that this interaction not only helps regulate the cell’s contractility, but also that vimentin intermediate filaments help regulate actin polymerization, promoting proper cell signaling.
“The findings are very basic but certainly reflect on many aspects of cell physiology and cell migration within developing tissue and organ systems as well as mechanisms responsible for normal cell movements within complex tissues,” said Goldman, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Goldman added that the findings could also have pathological implications, such as in the case of wound healing and the regulation of cell movement in cancer metastasis, both of which require proper function of a cell’s mechanical properties—supported by vimentin intermediate filaments—and contractility—enabled by F-actin and its associated proteins.
More information:
Huayin Wu et al, Vimentin intermediate filaments and filamentous actin form unexpected interpenetrating networks that redefine the cell cortex, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2115217119
Provided by
Northwestern University
Citation:
Cytoskeletal proteins interact to form intracellular networks (2022, April 8)