It’s estimated that mining, refining, and processing metals commonly used in construction, referred to as structural metals, contribute around three billion tons of CO2-equivalent emissions. And, although recycling these materials has the potential to mitigate their negative environmental impact, many sustain damage, like fractures, that can preclude them from extending their lifecycles.
High-temperature techniques, such as brazing and welding, have been used for metal repair for thousands of years. However, these, too, suffer from limitations in that certain alloys—metallic substances made up of two or more elements—are prone to cracking under extreme heat. Some new complex 3D printed structures are too intricate or delicate to access with these tools.
In a paper published in the journal Advanced Materials, a team of researchers led by James Pikul of the School of Engineering and Applied Science presents a novel technique to restore metals’ strength and toughness. The researchers have used this “electrochemical healing” to repair fractured metals in various metallic materials, including steel, aluminum alloys, and complex 3D printed structures, under room-temperature conditions.
“Metals that are difficult to repair usually end up as waste, causing both economic and environmental problems,” says Pikul, an assistant professor of mechanical engineering and applied mechanics. “Our electrochemical healing technique offers a solution to this by enabling the full recovery of the metal’s tensile strength, including ‘unweldable’ aluminum alloys used in aerospace. This opens up a whole new range of possibilities for repairing metals in a cost-effective and sustainable way.”
The lead author of the paper is Zakaria H’sain, a postdoctoral researcher in the Pikul Research Group who earned a Ph.D. from Penn. H’sain explains that brazing is similar to a dentist filling a tooth in that the cavity is sealed by inserting a filler material and funneling it into the weakened site; and welding can make two pieces of metal into one by melting them together with a high-temperature laser or spark. He says that their new metal healing technique uses a different approach to repair metals.
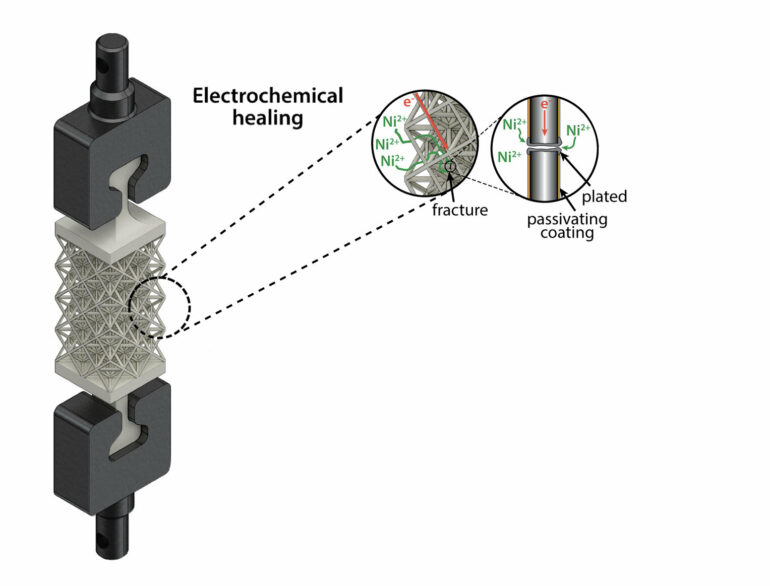
“We call our method electrochemical healing because it more closely resembles how our bodies repair a bone fracture,” H’sain says. “The healing matter is transported to the fracture site and strength is recovered through the growth and connection of matter from opposite fracture surfaces.”
To allow the metal to “heal,” the researchers placed it in a type of water-based solution known as electrolyte; theirs was a salty water mixture that contained nickel ions. The team then applied a negative voltage, which moved ions in the electrolyte toward the metal cracks, leading to an increase in electrons, until metal ions from the electrolyte started to steal excess electrons, a chemical reaction called a reduction. This turned the ions into solid metal atoms, and as atoms grew on the surface they healed the fracture.
“We applied a protective polymer coating to the metal to act as a barrier, so when it is exposed to the electrolyte nickel plating, or healing, it is limited to the fracture site and won’t interfere with any other parts of the metal structure,” H’sain says.
Building on their previous study investigating the possibilities of electrochemical healing for repairing metals, the team developed a model to gauge the efficacy of their repairs in restoring mechanical strength based on the geometry of the fracture, the original strength of the overall structure, the strength of the nickel coating, and other process parameters.
They applied their model to three different alloys: a relatively inexpensive low-carbon steel popular in construction and machinery and two “unweldable” aluminum alloys commonly used in aircraft wings and fuselages.
In a later experiment, the team collaborated with Masoud Akbarzadeh, assistant professor of architecture in the Stuart Weitzman School of Design, and Mostafa Akbari, a graduate student in Akbarzadeh’s Polyhedral Structures Laboratory, to see if electrochemical healing could be used to repair 3D printed efficient structures.
“We’ve shown we can recover 100% of the strength for all those alloys if we follow our model,” H’sain says. “Whereas previous electrochemical techniques have relied on elaborate chemical solutions tailored to each material, we present a one-size-fits-all approach that could be applied to many.”
“We’re particularly excited about the potential for electrochemical healing to revolutionize the repair of 3D-printed metal structures with complex morphology,” Akbarzadeh says. “By enabling full restoration of tensile strength in our difficult-to-weld shellular structure, we’re paving the way for more efficient and sustainable repair processes for these increasingly popular building materials.”
In future research, the team plans to expand upon their work with the 3D printed structure by designing and fabricating components that factor repairs needed beforehand to ensure effective recovery of strength is more easily facilitated. Additionally, they are also interested in investigating methods of autonomous repair and reducing costs with alternative electrodeposited metals.
More information:
Zakaria Hsain et al, Electrochemical Healing of Fractured Metals, Advanced Materials (2023). DOI: 10.1002/adma.202211242
Provided by
University of Pennsylvania
Citation:
Mending the unmendable: A new method for restoring fractured metals (2023, April 28)