The world would look very different without multicellular organisms—take away the plants, animals, fungi, and seaweed, and Earth starts to look like a wetter, greener version of Mars. But precisely how multicellular organisms evolved from single-celled ancestors remains poorly understood. The transition happened hundreds of millions of years ago, and early multicellular species are largely lost to extinction.
To investigate how multicellular life evolves from scratch, researchers from the Georgia Institute of Technology decided to take evolution into their own hands. Led by William Ratcliff, associate professor in the School of Biological Sciences and director of the Interdisciplinary Graduate Program in Quantitative Biosciences, a team of researchers has initiated the first long-term evolution experiment aimed at evolving new kinds of multicellular organisms from single-celled ancestors in the lab.
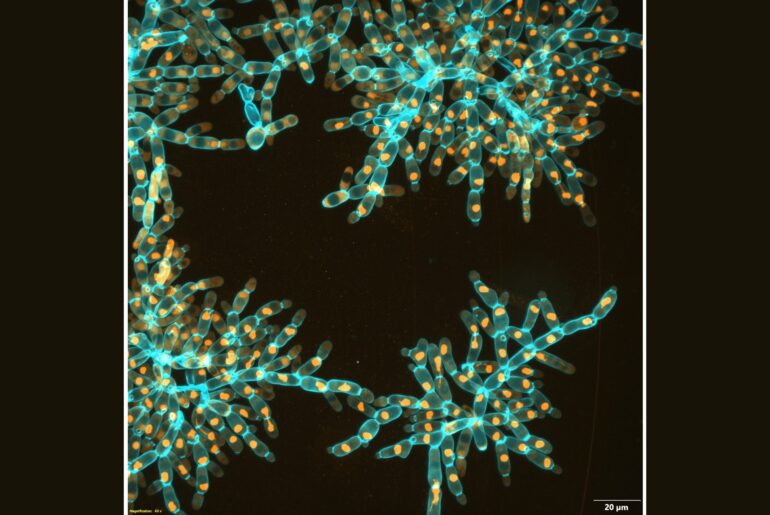
Over 3,000 generations of laboratory evolution, the researchers watched as their model organism, “snowflake yeast,” began to adapt as multicellular individuals. In research published in Nature, the team shows how snowflake yeast evolved to be physically stronger and more than 20,000 times larger than their ancestor.
This type of biophysical evolution is a pre-requisite for the kind of large multicellular life that can be seen with the naked eye. Their study is the first major report on the ongoing Multicellularity Long-Term Evolution Experiment (MuLTEE), which the team hopes to run for decades.
“Conceptually, what we want to understand is how simple groups of cells evolve into organisms, with specialization, coordinated growth, emergent multicellular behaviors, and life cycles—the stuff that differentiates a pile of pond scum from an organism that is capable of sustained evolution,” Ratcliff said. “Understanding that process is a major goal of our field.”
The multicellularity long-term evolution experiment
Ozan Bozdag, a research scientist and former postdoctoral researcher in Ratcliff’s group and first author on the paper, initiated the MuLTEE in 2018, starting with single-celled snowflake yeast. Bozdag grew the yeast in shaking incubators and each day selected for both faster growth and larger group size.
The team selected on organism size because all multicellular lineages started out small and simple, and many evolved to be larger and more robust over time. The ability to grow large, tough bodies is thought to play a role in increasing complexity, as it requires new biophysical innovations. However, this hypothesis had never been directly tested in the lab.
Over about 3,000 generations of evolution, their yeast evolved to form groups that were more than 20,000 times larger than their ancestor. They went from being invisible to the naked eye to the size of fruit flies, containing over half a million cells. The individual snowflake yeast evolved novel material properties: while they started off weaker than gelatin, they evolved to be as strong and tough as wood.
New biophysical adaptations
In investigating how the snowflake yeast adapted to become larger, the researchers observed that the yeast cells themselves became elongated, reducing the density of cells packed into the group. This cell elongation slowed down the accumulation of cell-to-cell stress that would normally cause the clusters to fracture, allowing the groups to get larger. But this fact alone should have only resulted in small increases in size and multicellular toughness.
To uncover the precise biophysical mechanisms that allowed growth to macroscopic size, the researchers needed to look inside the yeast clusters to see how the cells interacted physically. Normal light microscopes were unable to penetrate the large, densely packed groups, so the researchers used a scanning electron microscope to image thousands of ultrathin slices of the yeast, which gave them their internal structure.
“We discovered that there was a totally new physical mechanism that allowed the groups to grow to this very, very large size,” Bozdag said. “The branches of the yeast had become entangled—the cluster cells evolved vine-like behavior, wrapping around each other and strengthening the entire structure.”

Snowflake yeast clusters go from ~100 cells per cluster (left tube) to nearly half a million cells per cluster (right tube). © Georgia Institute of Technology
By simply selecting on organismal size, the researchers figured out how to leverage the biomechanical mechanism of entanglement, which ended up making the yeast about 10,000 times tougher as a material.
“Entanglement has previously been studied in totally different systems, mostly in polymers,” said Peter Yunker, associate professor in the School of Physics and a co-author on the paper. “But here we’re seeing entanglement through an entirely different mechanism—the growth of cells rather than just through their movement.”
Observing the entanglement was a turning point in the researchers’ understanding of how simple multicellular groups evolve. As a brand-new multicellular organism, snowflake yeast lacks the sophisticated developmental mechanisms that characterize modern multicellular organisms. But after just 3,000 generations of laboratory evolution, the yeast figured out how to drive and co-opt cellular entanglement as a developmental mechanism.
Preliminary investigations of other multicellular fungi show that they also form highly entangled multicellular bodies, suggesting that entanglement is a widespread and important multicellular trait in this branch of multicellular life.
“I’m really excited to have a model system where we can evolve early multicellular life over thousands of generations, harnessing the awesome power of modern science,” Ratcliff said. “In principle, we can understand everything that is happening, from the evolutionary cell biology to the biophysical traits which are directly under selection.”
For a long time, humans have worked with biology to evolve new things—from the corn we eat to domesticated dogs, chickens, and show pigeons. According to Ratcliff, what their team is doing is not so different.
“By putting our finger on the scale of a single-celled organism’s evolution, we can figure out how they evolved into progressively more complex and integrated multicellular organisms, and can study that process along the way,” he said. “We hope that this is just the first chapter in a long story of multicellular discovery as we continue to evolve snowflake yeast in the MuLTEE.”
More information:
Ozan Bozdag, De novo evolution of macroscopic multicellularity, Nature (2023). DOI: 10.1038/s41586-023-06052-1. www.nature.com/articles/s41586-023-06052-1
Provided by
Georgia Institute of Technology
Citation:
A journey to the origins of multicellular life: Long-term experimental evolution in the lab (2023, May 10)