The cells in our bodies are continuously exposed to mechanical forces that are either externally applied or generated by the cells themselves. Being able to respond to such mechanical stimuli is an indispensable prerequisite for a large number of biological processes.
However, how cells manage to process mechanical stimuli is poorly understood because techniques to study the very fine mechanical signals in cells are lacking. Researchers at the University of Münster (Germany) have now developed a method for altering the mechanics of individual molecules and thereby investigating their function within the cells. The findings have been published in the journal Science Advances.
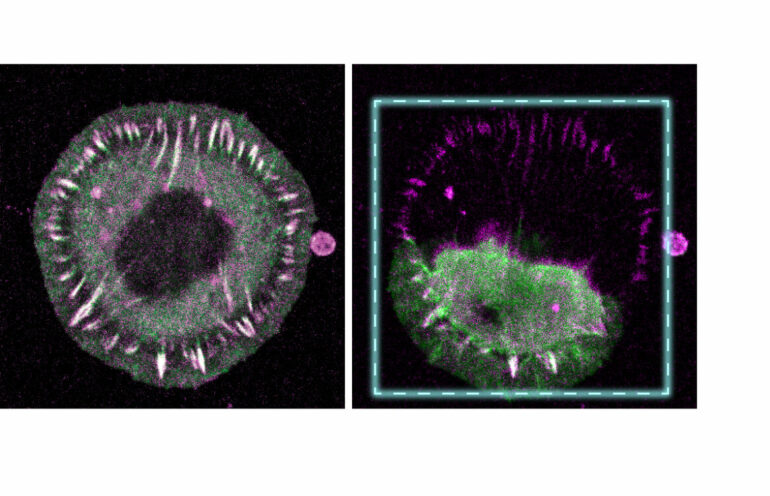
The team, headed by cell biologist Prof. Carsten Grashoff, developed a method in which proteins can be altered with the help of a light-sensitive molecule and mechanically controlled by short light pulses. In this way, the scientists succeeded in breaking individual proteins with high temporal and spatial control enabling them to investigate their mechanical significance in the cells.
Their first experiments demonstrated the function of two molecules that are not only important for the adhesion of cells but that are suspected of playing a central role in a number of diseases. The talin protein is essential for the carrying of mechanical forces during adhesion of cells in connective tissue—a process which is highly important in, for example, cell migration. In contrast, the desmoplakin protein is important for resisting mechanical stress in the cell-cell junctions that occur in epithelial tissues such as skin.
Mechanical stimuli, like many other signals, are ultimately processed in cells at the level of individual proteins. Although researchers have, over the past few years, identified a range of molecules that are directly exposed to mechanical forces in cells, it has often remained unclear how important the mechanical contributions of individual proteins are in these often very complex cell-biological processes.
The experiments carried out by Grashoff’s team succeeded by utilizing a light-sensitive connection, that despite being able to withstand a high degree of mechanical forces, breaks down when exposed to light radiation. Comparable light-sensitive proteins are found in plants, where they regulate the plant’s orientation to light. By inserting these predetermined breaking points into specific genes (talin, desmoplakin) using molecular biology techniques, the team produced cells of connective tissue and skin that could be controlled with a laser beam at the level of individual proteins. Modulation and analysis of the living cells, derived from mouse cell culture models, were achieved with fluorescence microscopy methods.
“Together, these results provide evidence about how the mechanical properties of certain cell structures can be controlled by individual proteins,” says Grashoff.
As the developed technique is genetically encoded, and can therefore be inserted at any point into the genome, the researchers hope it will have broad applicability in the investigation of the mechanobiological properties of many other proteins in living cells, model organisms and disease models.
More information:
Tanmay Sadhanasatish et al, A molecular optomechanics approach reveals functional relevance of force transduction across talin and desmoplakin, Science Advances (2023). DOI: 10.1126/sciadv.adg3347
Provided by
University of Münster
Citation:
Demonstrating the significance of individual molecules during mechanical stress in cells (2023, June 21)