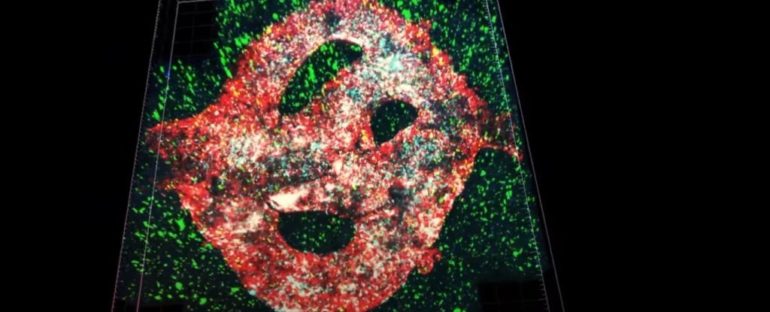
For the first time, one of the deadliest forms of brain tumor has been successfully 3D-bioprinted, resulting in the most complete lab-grown model yet.
Scientists from Tel Aviv University printed a glioblastoma in a brain-like environment, including vessels that supply the mass with blood. This is the most complete replication of a tumor and surrounding tissue yet – a breakthrough that could help develop treatments, the researchers said.
Glioblastoma may be rare, but it’s particularly horrible. It grows quickly and aggressively on the brain or brain stem, cannot be cured, and is almost always fatal.
It’s also hard to treat. Because the cancer is so aggressive, treatment needs to be pretty hardcore, usually requiring courses chemotherapy and radiotherapy, which the patients often grow too ill to complete.
Glioblastoma tissue, taken and cultured from tumors removed from patients, is one avenue via which doctors hope to learn more about this diabolical cancer. This is usually done on petri dishes, and is an extremely useful tool – but it has limitations, said cancer researcher and nanoscientist Ronit Satchi-Fainaro of Tel Aviv University.
In a previous study, she and her team had found a protein called P-Selectin that is produced when cancer cells in glioblastoma encounter microglial cells in the brain – the most prominent immune cells in the central nervous system.
This protein triggers the microglia to act in support of the glioblastoma, rather than fighting against it – with devastating results for the person.
“However, we identified the protein in tumors removed during surgery, but not in glioblastoma cells grown on 2D plastic petri dishes in our lab,” she explained.
“The reason is that cancer, like all tissues, behaves very differently on a plastic surface than it does in the human body. Approximately 90 percent of all experimental drugs fail at the clinical stage because the success achieved in the lab is not reproduced in patients.”
The team’s attempt at finding a solution to this limitation was a glioblastoma bio-ink, created from glioblastoma cells, astrocytes, and microglia derived from a patient. Using a removable bio-ink coated in types of cells that form blood vessels, they also managed to provide their model with a functional blood supply.
Each glioblastoma model was 3D-printed in a bioreactor in a hydrogel based on an extracellular matrix also taken from the patient.
The glioblastoma model was then connected to and communicated with the extracellular matrix via the blood vessels, to simulate the way the tumors interact with the surrounding brain tissue. This provides a way to study the way the cancer behaves that is specific to its environment – the brain.
“The physical and mechanical properties of the brain are different from those of other organs, like the skin, breast, or bone,” Satchi-Fainaro said.
“Breast tissue consists mostly of fat, bone tissue is mostly calcium; each tissue has its own properties, which affect the behavior of cancer cells and how they respond to medications. Growing all types of cancer on identical plastic surfaces is not an optimal simulation of the clinical setting.”
The team then tested their models using P-Selectin. A P-Selectin inhibitor was introduced to glioblastoma cultures grown in petri dishes, as well as the 3D-printed models and animal models. In the petri dish cultures, no change was observed in the growth or cell migration, compared to untreated controls.
For the 3D-printed and animal models, the P-Selectin inhibitor resulted in a slower growth rate compared to untreated controls.
“This experiment showed us why potentially effective drugs rarely reach the clinic simply because they fail tests in 2D models, and vice versa: why drugs considered a phenomenal success in the lab, ultimately fail in clinical trials,” Saitchi-Fainaro said.
Genetic sequencing and the growth rate of the 3D-printed tumors also more closely matched what the team observed in living patients. On 2D petri dishes, the samples change over time so that they no longer match the patients’ tumors, but the 3D-printed glioblastomas remained similar to patient samples.
In addition, the 2D cultures all grow at the same rate; whereas the 3D-printed tumors showed varying growth rates, which is what is observed in humans and animals.
This not only suggests a way to more accurately study the behavior of glioblastoma, it could lead to ways to develop patient-specific interventions.
“If we take a sample from a patient’s tissue, together with its extracellular matrix, we can 3D-bioprint from this sample 100 tiny tumors and test many different drugs in various combinations to discover the optimal treatment for this specific tumor,” Saitchi-Fainaro explained.
“But perhaps the most exciting aspect is finding novel druggable target proteins and genes in cancer cells – a very difficult task when the tumor is inside the brain of a human patient or model animal.
Our innovation gives us unprecedented access, with no time limits, to 3D tumors mimicking better the clinical scenario, enabling optimal investigation.”
The research has been published in Science Advances.