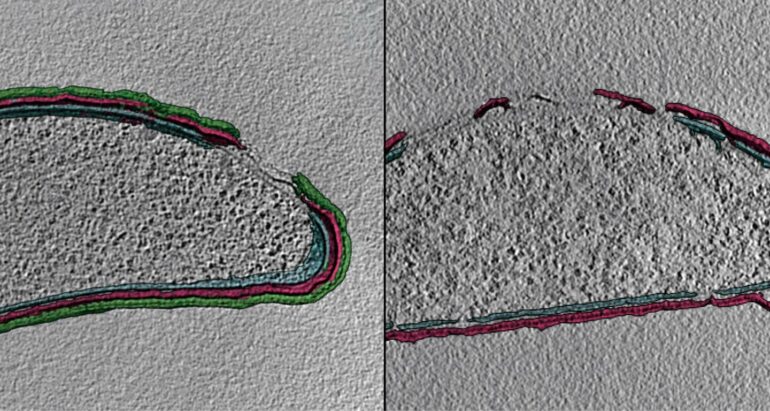
Cells are the basic units of life—but many of their fundamental processes happen so fast and at such small length scales that current scientific tools and methods can’t keep up, preventing us from developing a deeper understanding.
Now, researchers with SLAC National Accelerator Laboratory, Stanford University, Cornell University and other institutions have developed a new approach for watching basic biological processes unfold. The approach, which combines cryogenic electron microscopes with methods developed in X-ray crystallography, could lead to improved medicines and a deeper understanding of cell division, photosynthesis and host-pathogen interactions, among other subjects.
Their study is published in the journal Molecular Biology of the Cell.
“Many cellular processes happen on a millisecond timescale,” SLAC scientist and paper co-author Pete Dahlberg said. “With our new technique, we can poke a cell and then pick a moment in time that we want to snap a clear image of its response.”
Reimagining a powerful spray tool
For many decades, scientists have relied on imaging techniques known as cryogenic electron microscopy (cryo-EM) and cryogenic electron tomography (cryo-ET) to see inside of cells, proteins, and other organisms and molecules. Both techniques use electron microscopes to capture snapshots of flash-frozen samples, which have revealed cellular structures in extraordinary detail.
These approaches involve putting a sample on a thin small disk known as an electron microscopy grid and plunging it into a cryogenic liquid to freeze it very rapidly. This is great at preserving cellular samples in their native state, but the frozen snapshots don’t tell researchers much about dynamics. It is sort of like trying to learn dance moves by taking random images of someone dancing.
Currently, in similar cryo-ET experiments, researchers hand-mix cell samples in order to take images of them in response to stimuli. But hand-mixing takes time, kind of like mixing pancake batter by hand instead of with an electric mixer, meaning that experimenters can only observe changes in an organism at about ten second intervals—hundreds of times longer than many important processes take.
“When you hand-mix and freeze cells in cryo-ET experiments, you are often too slow to capture the changes you really care about. That can limit your ability to understand important biological processes,” SLAC researcher and paper co-author Cali Antolini said.
Researchers therefore turned to a spray nozzle device that is often used at X-ray free-electron laser (XFEL) and synchrotron facilities to mix samples for crystallography experiments. The device, known as a mixing injector coupled Gas Dynamic Virtual Nozzle (GDVN), is often used to study molecular movements that occur on extremely short timescales, like femtoseconds after activation with light or on millisecond to second timescales using chemical mixing, at XFELs like SLAC’s Linac Coherent Light Source (LCLS).
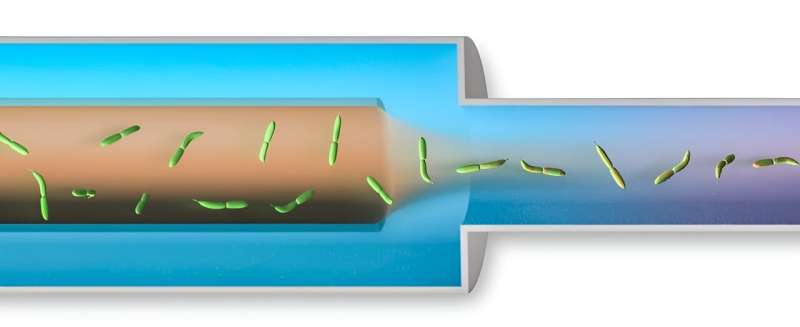
A graphic representation of the spray nozzle device. The sample cells (green) mix with the simulant solution as the cells travel from left to right, out of the spray nozzle. © Greg Stewart/SLAC National Accelerator Laboratory
“The spray device at LCLS allows researchers to look at the movements of atoms in microcrystals,” Dahlberg said. “But to my mind, spraying microcrystal samples and spraying cell samples are the same thing.”
“We wanted to combine light source and cryo-ET techniques as much as possible,” SLAC and Stanford professor and senior co-author Soichi Wakatsuki said. “We knew that doing so would be fruitful for microbiology and medicine development.”
With their new approach, researchers sprayed and froze cell samples that had been mixed with a stimulant in milliseconds, rather than 10 seconds, the time hand-mixing takes. This allowed researchers to take images of the cell sample every 25 milliseconds and see changes on that time scale.
“Our new approach helped to identify and characterize some of the interesting morphological changes in the cells that we began to see over the course of our time-resolved experiments,” Stanford University graduate student and paper co-author Jacob Summers said.
From jet to mist
Researchers from Cornell University re-engineered LCLS’s spray nozzle to work for the cryo-ET experiment. But it wasn’t as simple as walking the spray device from LCLS over to a cryo-ET machine. The problem was that, at LCLS, the samples are sprayed in a powerful jet formation—like a garden hose set on jet. This force and pressure would not work for cryo-ET experiments because samples are sprayed onto a thin, fragile grid surface, which would probably break under the force of a jet stream.
Therefore, researchers adjusted the gas flow rate through the nozzle—kind of like changing the setting on a garden hose from jet to mist. With this adjustment, they created a fine spray rather than a powerful stream.
Since this was a relatively new technique, the correct conditions for creating a misty spray were virtually unexplored, said Kara Zielinski, a researcher at Cornell and paper co-author. They had to test a lot of different experimental conditions, like the liquid flow rate, gas flow rate, distance of the sprayer to the grid, and even the grid type to find the optimal conditions for high quality grids and data collection, she said.
The researchers also varied the flow rates of cell and stimulant solutions within the spray device, in effect controlling how fast a sample mixed together and starting the clock for the cellular reactions researchers want to study.
Now that the researchers have proven this new technique, it could be applied to a wide variety of questions about structural dynamics on the cellular level, Zielinski said.
“It is always exciting to be part of the beginning of a new method because it often means opening up entirely new avenues of biological questions,” she said. “The opportunities are endless as we can now trigger cellular dynamics by mixing in small molecules and capture direct structural evidence of its effects.”
“I’m most excited about what this method could lead to in the future,” said Joey Yoniles, paper co-author and Stanford University graduate student. “Even if we just consider bacteria like we did in this study, we could look at the interaction between bacteria and drugs at extremely high resolution.”
More information:
Joseph Yoniles et al, Time-resolved cryogenic electron tomography for the study of transient cellular processes, Molecular Biology of the Cell (2024). DOI: 10.1091/mbc.E24-01-0042
Provided by
SLAC National Accelerator Laboratory
Citation:
A novel spray device helps researchers capture fast-moving cell processes (2024, June 6)