An important question in modern life science and medical research is how the microorganisms living in and on a body influence the life processes and thus health and disease of the host organism. Scientists assume that there are connections between the entirety of the body’s microbial colonization, the so-called microbiome, and the development of diseases.
Chronic inflammatory bowel disease (IBD) in particular is apparently closely linked to the composition and disturbance of the microbiome. At the same time, it is difficult to define a healthy normal state of the human microbial colonization, as it is influenced by many factors and the individual composition varies from person to person.
Scientists from the Collaborative Research Center (CRC) 1182 “Origin and Function of Metaorganisms” in Kiel have now compared microbiome data from various great apes with those of humans with rural and urban lifestyles in the largest study of its kind to date, in order to identify certain patterns of similarities and differences in the microbial colonization of the various hosts.
The researchers hope to gain new insights into the influence of evolutionary development, the environment and lifestyle on the composition of the microbiome and identify possible effects. In their new work, they were able to confirm above all that the microbial colonization of a living organism is very host-specific, that the evolution of microorganisms and their hosts takes place together and in parallel, and that the species diversity of the human microbiome is reduced in comparison with apes.
Together with researchers from various partner institutions, including the Helmholtz Institute for One Health in Greifswald, a subdivision of the Helmholtz Center for Infection Research, the team recently published their findings in the journal Nature Communications.
Metagenome study analyzes data from great apes and humans
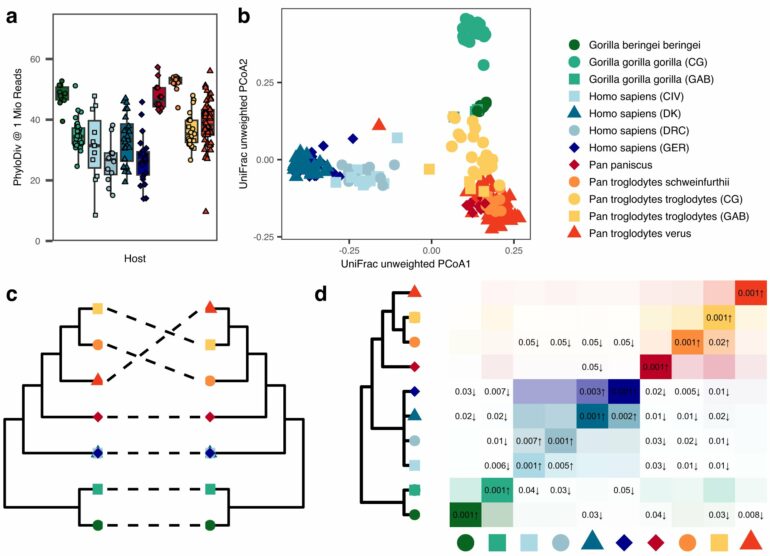
The CRC 1182 research team conducted their so-called metagenome study using about 200 stool samples from wild African great apes, including chimpanzees and gorillas, and from human subjects from the Democratic Republic of Congo and Côte d’Ivoire as well as from Denmark and Germany. The African subjects live in rural environments in vicinity of the national parks where the animals were sampled, while the European participants come from urban environments.
The genomes of microorganisms obtained in this way represent the largest data set of its kind to date. In this way, the scientists were able to determine the diversity and composition of the microbial species contained in the respective microbiomes and compare them in terms of their development and the impact of environmental influences.
“We were particularly interested in the evolutionary perspective, i.e., how microbiomes have developed from a common ancestor to today’s ape species and human populations over long periods of time,” says Dr. Malte Rühlemann, first author of the study and research associate in Professor Andre Franke’s working group at the Institute of Clinical Molecular Biology (IKMB).
“We hypothesize that this approach can also provide us with insights into the development of diseases that are probably influenced by the microbiome, for example, chronic inflammatory bowel diseases.”
The microbiome follows the evolution of the host
An initial result of this comparative study was that it was once again able to confirm the high host-specificity of the microbiome—in other words, that the microbial colonization of the intestine in each host organism has a very characteristic composition. “We were able to clearly differentiate between the microbiome of a chimpanzee, gorilla or human, for example, based on the different bacterial species and their proportions,” says co-author Dr. Jan Gogarten from the Helmholtz Institute for One Health.
These characteristic microbiomes can be clearly distinguished from each other by the absence or presence of certain key species and their composition follows the evolutionary trajectory of their hosts in terms of their similarity. Researchers refer to such a joint evolution of host and symbiont as phylosymbiosis.
“Our data show clear signs of this. In all the great apes we studied, we found signals that indicate conserved evolutionary relationships between microbial communities and their host species. This underpins the importance of phylosymbiosis as a result of close interaction between host and microbiome over evolutionary time,” says Dr. Gogarten.
Functional analyses provide insights into the effects of microbiome changes
The comparison of microbiome compositions and changes in different hosts also raises the question of how their differences have a functional impact and what role certain individual microbial species or groups play in this. In particular, the study was able to show that some evolutionarily conserved microorganisms are successively lost in people with urban lifestyles.
As a striking example, the Kiel research team analyzed the group of Prevotella bacteria. “These are found very rarely in European microbiomes, but occur more frequently in the samples from the Democratic Republic of Congo and Côte d’Ivoire and on a massive scale in animal hosts. We therefore suspect that this group of bacteria has been an integral part of the hominid microbiome for millions of years from an evolutionary perspective, but is significantly reduced in the human gut in connection with an urban lifestyle,” explains Dr. Corinna Bang, co-author and head of the microbiome laboratory at the IKMB.
To find out what these changes could mean at a functional level, the researchers analyzed how the Prevotella bacteria found in the human microbiome differ genetically from their conspecifics in the intestines of chimpanzees. They discovered that within this bacterial genus, a specific gene is present in 90% of bacteria associated with humans, but in none of the species found in apes.
“So far, this gene has not been studied in detail in Prevotella. However, experiments with the model bacterium Escherichia coli show that it enables the microorganisms to react very sensitively to oxygen and possibly helps the bacteria to survive under conditions that are not completely oxygen-free, such as those that occur temporarily in the human intestine,” says Bang.
One explanation for this is that it could be a functional adaptation of the bacteria to life specifically in the human intestine. However, the long-term presence of oxygen in the gut is often associated with inflammatory processes, particularly in IBD, where there is a massive increase in the intestinal oxygen level.
While cause and effect have not yet been sufficiently clarified, this genetic adaptation and ultimately the disappearance of Prevotella bacteria may be linked to disease risks, which the researchers want to investigate in more detail in the future.
“Overall, our study provides important new insights into the relationships between the effects of an urban lifestyle, the loss of evolutionarily conserved bacterial groups and the resulting possible functional adaptations of the human microbiome,” says Professor Andre Franke, last author of the study and board member of the PMI Cluster of Excellence.
“Although we are still unable to conclusively attribute diseases such as IBD to these factors, we now have convincing further evidence that renders disturbances and changes in the human microbiome plausible mechanisms of disease development. We want to further research these relationships to allow prophylactic or therapeutic interventions in microbiome-associated diseases in the future,” says Franke, who will continue to lead several projects in the CRC 1182 over the next four years.
More information:
M. C. Rühlemann et al, Functional host-specific adaptation of the intestinal microbiome in hominids, Nature Communications (2024). DOI: 10.1038/s41467-023-44636-7
Citation:
Comparative genome study of humans and great apes provides insight into development of gut microbiome (2024, January 15)