A team of CiRA researchers has uncovered the crucial role of EIF3D—a protein translational regulator—in primed pluripotency. The research is published in the journal Science Advances.
According to the central dogma of molecular biology, information flows from DNA to RNA to protein. While much is known about pluripotency—the ability to differentiate into any other cell type in the body and to divide indefinitely—in terms of transcriptional and epigenetic regulation, as well as signal transduction, how protein translation ties these control mechanisms together remains largely underexplored.
To identify genes important for maintaining primed pluripotency—a state poised for differentiating into various cell types in the body, the research team, led by Associate Professor Kazutoshi Takahashi and Assistant Professor Chikako Okubo, began with a genome-wide genetic screen based on CRISPR interference (CRISPRi) that systemically reduces the expression of every single gene in the genome of a pluripotent stem cell (PSC) line.
By monitoring a standard marker of undifferentiated cells at different time points after shutting down gene expression, the researchers can determine whether a specific gene positively or negatively regulates pluripotency.
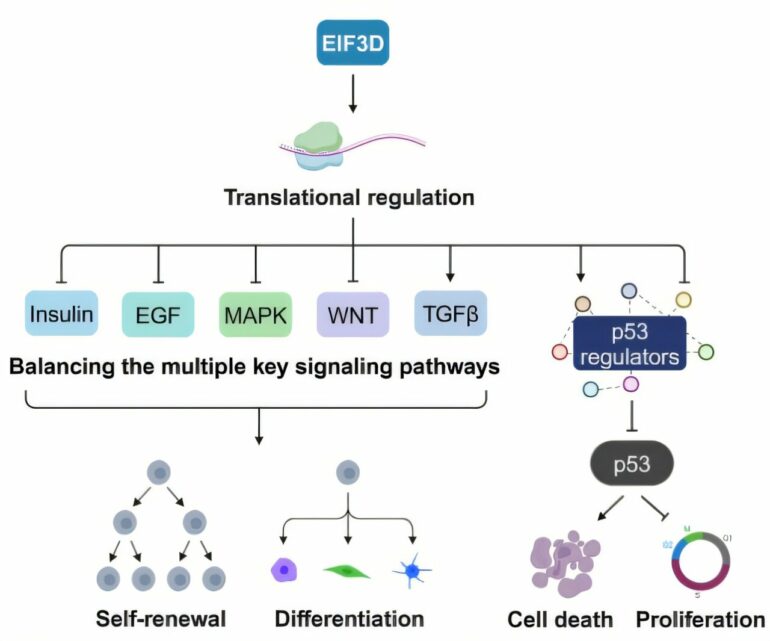
Notably, they discovered genes associated with protein translation to influence pluripotency substantially. In particular, they focused on EIF3D, which dramatically reduced cells with primed pluripotency and belongs to the EIF3 family that interacts with EIF4G2, a translational regulator known to maintain primed pluripotency.
Using an induced pluripotent stem (iPS) cell line with suppressed EIF3D expression, the researchers also observed morphological changes and growth defects. Further analysis showed that these cells had reduced gene expression of transcription factors promoting pluripotency.
Conversely, the transcriptional targets of p53—a cell cycle regulator often mutated in cancers—such as CDKN1A and MDM2—were upregulated in these cells. In addition, markers of cellular stress and senescence were induced.
Remarkably, the protein expression of p53 was significantly increased in cells with suppressed EIF3D expression despite unchanged expression at the RNA level, suggesting that reduced EIF3D leads to the translational dysregulation of p53.
To further investigate the role of EIF3D in maintaining pluripotency, the research team examined the gene expression profile of cells with suppressed EIF3D expression, cells with suppressed core pluripotency transcription factors, and cells differentiated into the three major cell germ layers.
Cells with reduced EIF3D lacked strong marker expression related to primed PSC or germ line cells, thus assuming a state different from other cells in the comparison.
When the research team specifically examined messenger RNAs (mRNAs) being translated in cells with suppressed EIF3D expression, they determined that EIF3D is involved in directing the binding of protein translation machinery to certain mRNAs in primed PSCs.
Furthermore, many genes associated with pluripotency were downregulated in its absence, thus affecting multiple signaling cascades, including the p53 pathway, that regulate primed pluripotency.
Through this work, the research team revealed the critical role of EIF3D in safeguarding primed pluripotency. Moreover, it further highlights the impact of translational regulation on pluripotency.
By improving our understanding of factors controlling pluripotency, this discovery helps to propel the basic study of stem cells and their practical application for next-generation medicine purposes.
More information:
Chikako Okubo et al, EIF3D safeguards the homeostasis of key signaling pathways in human primed pluripotency, Science Advances (2025). DOI: 10.1126/sciadv.adq5484
Citation:
CRISPR screen identifies EIF3D as critical regulator of stem cell pluripotency maintenance (2025, April 11)