It’s a cold, wet day in 2015 and Anne Pringle is scouring the understory of a Northern California forest for the unassuming organism that has consumed her research for the last several years: the death cap mushroom, or Amanita phalloides.
This fungus isn’t the whimsical, polka-dotted toadstool of childhood cartoon nostalgia, but the fatally toxic mushroom that has invaded the North American West Coast. Pringle and her lab have been collecting specimens to try and determine how the death cap has been able to invade this environment.
Little did she know that eight years later, she and a team of researchers from the University of Wisconsin–Madison and the U.S. Department of Agriculture’s Agricultural Research Service (USDA-ARS) would be the first to document some of the complexity behind that mystery.
In a paper published this week in The ISME Journal, the team found that individual death cap mushrooms seem to have their own unique suite of toxin genes.
“In other words, not all mushrooms are poisonous in the same way,” says Pringle, who is a professor of botany at UW–Madison.
The discovery could help researchers better understand how the toxic fungus is successfully invading California and open the door for new drug discovery pathways.
Pringle, an evolutionary ecologist, was the first to determine that the population of death caps found in California is not native, as scientists previously thought; instead, the infamous fungus is an invasive species. Pringle wondered if part of the mushroom’s invasion success lay in the toxins it’s so well-known for. That meant she needed to get a better understanding of the genes that create these toxins.
Pringle teamed up with UW–Madison Medical Microbiology and Immunology professor Nancy Keller; former postdoctoral researcher Milton Drott; and Sung Chul Park, a current postdoctoral researcher in Keller’s lab.
Keller and her lab focus on picking apart the genetic reasons behind why a fungus, such as the carcinogenic Aspergillus flavus, may be a potent pathogen. They also study the potential for compounds isolated from pathogenic fungi to lead to new drug discovery.
“We’re always looking for new compounds that could be useful for medicinal purposes,” Keller says. “We can take these new purified metabolites and say, ‘Oh, do they impact growth of harmful microbes?’ You just don’t know until you test.”
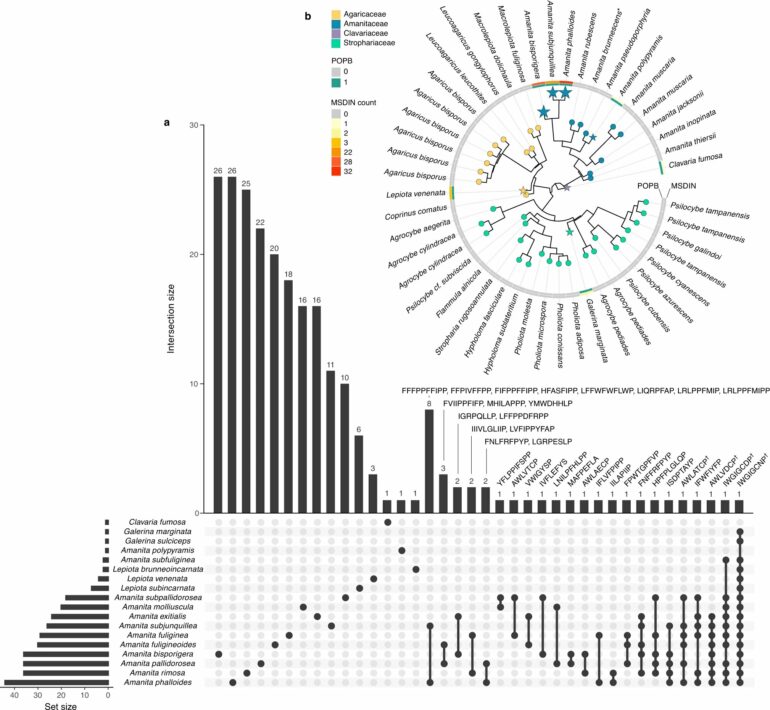
For the new study, Drott, who now works for the USDA-ARS, relied on his background in computational biology to analyze genetic data from samples of death cap mushrooms collected in California and Europe. The team was looking to document the genes underpinning their toxicity.
What the researchers discovered was an unexpected diversity of such genes.
All the specimens share a core group of genes regardless of their origin. Having these core genes is generally thought of as a requirement for a specimen to be classified as part of the death cap species, explains Drott.
But the researchers also found an accessory group of genes. From individual to individual, some mushrooms had certain accessory genes while others were missing those same genes entirely.
It’s like if everyone at a lakeside barbecue had a citronella candle to help fend off pesky bugs. The citronella candle is like a core gene, emitting a smell to hopefully keep the bugs at bay. Now, if that lakeside turned into a swamp-side, the barbecue goers might have to adjust their bug-repelling strategy.
In addition to their citronella candle, some people may bring bug spray, others might set up a mosquito trap, maybe some try a natural bug repellent oil. These extra bug repellent methods are like accessory genes, varied methods the barbecue goers are trying out to see what works best in the new location.
In the mushrooms, Pringle believes these accessory genes create a variety of compounds, some of which may enable them to thrive best in new ecosystems and continue their invasion on the West Coast.
“We’re documenting evolution, there’s no doubt about that,” Pringle says.
But it’s a really difficult question for researchers to test.
In humans it’s easy to trace the heritability of genes from parents to offspring, since people typically get two sets of genes, one from each parent. But in mushrooms it’s much more complex. Sometimes individual mushrooms end up receiving a few versions of a gene. That makes it tricky to determine whether certain toxin genes play a role in helping a mushroom thrive in a particular environment.
“Computationally, that’s very hard to deal with because you have to differentiate which one came from which parent or if it’s altogether a unique sequence,” Drott says. “Teasing that apart was a major step forward in our ability to identify these toxins on this scale.”
Death cap mushrooms are also mycorrhizal, meaning they rely on plant roots to grow. That adds another layer of difficulty in designing experiments since it can be difficult or even impossible to recreate these conditions in the lab.
And, the researchers note, the mere presence of genes doesn’t always mean the compounds they’re producing are definitely all toxins.
“When you’re picking a mushroom, it’s a moment in time. So, it could be at that moment the gene hasn’t even been expressed and the compound not made,” Keller says. “We imagine that some of these genes are turned on due to stress. So ideally, you’d want to sample at different conditions.”
The team also notes that scientists don’t yet know why the death cap mushroom is toxic in the first place: Is it for self-defense? Are mushrooms territorial? Do toxins and other mushroom compounds vary in different geographic locations for different reasons? Finding the answers to these questions isn’t straightforward.
However, the team is excited by the findings and the opportunity to add to the under-researched literature of fungal invasion biology.
This is the first time that scientists have documented that there is so much variation between the native and invasive populations of death caps and where in the genome the variation exists, Drott says.
“There’s something happening on the West Coast, where [the mushroom] is spreading,” he says. “Every year, people eat these mushrooms and die. And we want to stop that if we can.”
More information:
Milton T. Drott et al, Pangenomics of the death cap mushroom Amanita phalloides, and of Agaricales, reveals dynamic evolution of toxin genes in an invasive range, The ISME Journal (2023). DOI: 10.1038/s41396-023-01432-x
Provided by
University of Wisconsin-Madison
Citation:
Death cap mushroom’s invasion success may be linked to newly documented variability of toxin genes (2023, May 26)