by Mohammad Ashhar I. Khan and Venkata R. Chirasani
Cells, the fundamental building blocks of life, are constantly subjected to a variety of mechanical forces within our bodies. These forces, which can arise from both internal and external sources, play crucial roles in regulating cellular processes such as migration, differentiation and tissue development.
As a research team captivated by the intricate workings of cells, we have always been driven by the fundamental question of how cells perceive and respond to these mechanical stimuli. In a study published in Nature Communications, our collaborative team from the University of North Carolina at Chapel Hill, Duke University and Penn State College of Medicine reveals significant progress made in unraveling the molecular mechanisms that enable cells to interpret and react to the mechanical forces they encounter.
The bustling city of the cell
Imagine a cell as a bustling city, with proteins serving as the workers that keep the city functioning. Just as a city must adapt to the forces of nature, such as wind and rain, cells must adapt to the mechanical forces they experience within our bodies. These forces can come from various directions and play crucial roles in processes such as cell migration, tissue development and wound healing.
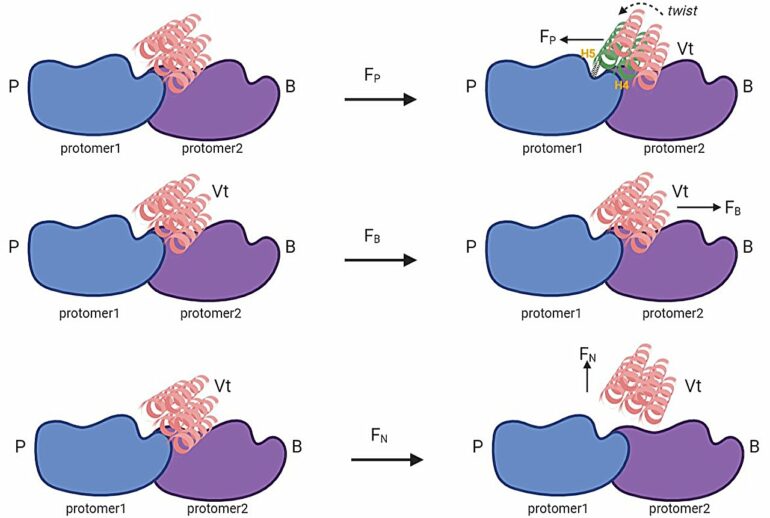
Our study focused on a protein called vinculin, which acts as a key connector between the cell’s internal skeleton (cytoskeleton) and its external environment. We used a powerful combination of computational modeling, protein engineering and advanced imaging techniques to investigate how vinculin senses and responds to directional forces.
To better understand vinculin’s role in directional force sensing, consider a dock-lock-latch mechanism using a spring model. Imagine a boat (representing actin) approaching a dock (representing vinculin) with a spring-loaded latch. As the boat comes in from one direction, it compresses the spring, allowing the latch to engage and secure the boat. However, if the boat approaches from the opposite direction, the spring doesn’t compress in the right way, and the latch doesn’t engage.
Similarly, vinculin acts like this directional latch mechanism. When forces are applied in a specific direction, vinculin forms stronger bonds with actin filaments, much like the latch securing the boat. However, when forces come from other directions, these strengthened interactions don’t occur, similar to how the latch wouldn’t engage if the boat approached from the wrong direction.
This directional force sensing ability of vinculin allows cells to distinguish and respond to mechanical forces coming from different directions, which is crucial for processes like cell migration and tissue development.
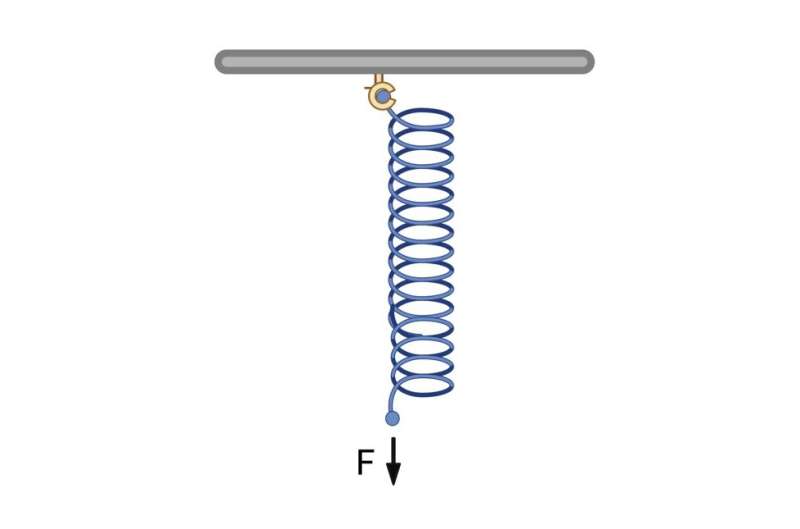
Spring-Loaded Latch Model of Vinculin’s Directional Force Sensing. This simple mechanical model helps illustrate how vinculin can act as a molecular force sensor, strengthening its interactions in response to forces applied in specific directions, much like the latch engaging more firmly when the spring is stretched downward. © Mohammad Ashhar I. Khan, Venkata R. Chirasani, University of North Carolina at Chapel Hill
Discovering the DAFS residues
Through our innovative approach, we discovered specific amino acid residues in vinculin that form directionally asymmetric force-strengthening (DAFS) interactions with actin filaments, the primary structural component of the cytoskeleton. These DAFS residues act like tiny sensors, allowing vinculin to detect and respond to forces applied in different directions.
To test our findings, we created vinculin variants lacking these DAFS residues and studied their effects on cellular behavior. Remarkably, cells expressing these modified vinculins exhibited impaired coordination between focal adhesions (the cell’s anchor points) and the actin cytoskeleton, leading to defects in the distribution of mechanical loads and the cells’ ability to migrate in a directed manner.
The implications of our work extend beyond basic science. By understanding how cells sense and respond to directional forces at the molecular level, we can gain valuable insights into processes such as tissue engineering, where precise control over cell behavior is crucial. Additionally, our findings may shed light on the mechanisms behind diseases such as cancer, where mechanical forces play a role in tumor progression and metastasis.
Potential applications and future directions
As we continue to explore the complex world of cellular mechanotransduction, we are excited about the potential applications of our research. By harnessing the power of computational modeling and experimental biology, we can develop novel approaches to manipulate cell behavior and create innovative therapies for a wide range of diseases.
Our research has provided new insights into the molecular basis of directional force sensing in cells, highlighting the critical role of vinculin and its DAFS residues. As we continue to push the boundaries of our knowledge, we are optimistic that our findings will contribute to the development of new strategies for controlling cell behavior and ultimately improving human health.
This study is just the beginning of our journey to unravel the secrets of how cells interact with their mechanical environment. I look forward to further collaborations with my colleagues in the field as we work toward a deeper understanding of these fundamental biological processes.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about Science X Dialog and how to participate.
More information:
Venkat R. Chirasani et al, Molecular basis and cellular functions of vinculin-actin directional catch bonding, Nature Communications (2023). DOI: 10.1038/s41467-023-43779-x
Dr. Mohammad Ashhar I. Khan is a Research Associate at the University of North Carolina at Chapel Hill, specializing in protein biochemistry, biophysics, and cell biology. With more than seven years of extensive hands-on experience in biologics research, Dr. Khan’s work focuses on elucidating the roles of cell adhesion proteins in cellular mechanics and disease processes. He holds a Ph.D. in Protein Biochemistry and Biophysics from the Indian Institute of Technology-Delhi and has published in high-impact journals including Nature Communications and the Journal of the American Chemical Society. Dr. Khan’s innovative research on protein–protein interactions and cellular mechanotransduction contributes significantly to our understanding of fundamental biological processes and holds promise for advancements in fields ranging from cancer research to regenerative medicine.
Citation:
Deciphering the language of cells: How they sense and respond to mechanical forces (2024, October 21)