Throughout their lives, cells encounter environments that vary in terms of how stiff or soft they are. These mechanical conditions impact just how quickly cells can grow, move and carry out basic functions like repairing damaged tissue. Though scientists have long known that cells can sense and respond to different environmental conditions, what isn’t clear is whether cells hold onto memories of those past conditions and whether previous experiences might lend them some advantage in future growth.
José Almeida, a graduate student in the Department of Biomedical Engineering, and Jairaj Mathur, a graduate student in the Department of Mechanical Engineering & Materials Science, working in the lab of Amit Pathak, associate professor of mechanical engineering & materials science in the McKelvey School of Engineering at Washington University in St. Louis, set out to test whether cells retain any memory of their past environments. And, if so, how much does that experience enhance their navigation of new, three-dimensional extracellular matrices, the protein-rich networks that surround cells and other body tissues?
Almeida and Mathur found that cells can store mechanical memory of past environments, but, more powerfully, cells also transfer that memory to the extracellular matrix surrounding them. In this way, cells effectively reshape their environments to share what they’ve learned with future cells, via the matrix rather than individual memory, easing the way for future invasions. Encoding the memories externally also ensures the preservation of collective memory beyond the life of a single cell in much less time than similar adaptations could be propagated through gene activation and epigenetic remodeling. These results were published May 5 in the journal Molecular Biology of the Cell.
“This question of whether cells exhibit memory-dependent behavior is a truly divisive topic for biologists,” Pathak said. “Some people view cell migration as all about adaption to environments—it doesn’t matter where you were before; the entire quest for motion is adaptation. Others would say that of course cells have memory. Cells are complex sets of genes and proteins, and, once they’ve established a network, the network’s not simply going to unravel the moment a cell leaves it.”
“What we’re learning is that both views are important, and there’s not one clear answer, particularly as it pertains to cell migration,” Pathak added. “Cells do adapt, but they also remember. Then the question becomes, which parts are memory dependent and which are more adaptation dependent?”
To test cellular memory and its role in cell migration, Almeida and Mathur combined their expertise in biomedical engineering and computational biophysics.
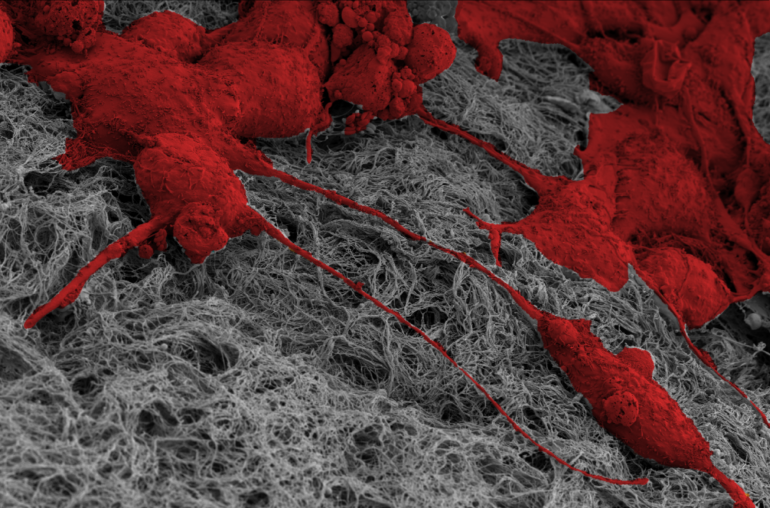
Almeida implanted cells previously exposed to stiff matrices into various 3D collagen environments. These “stiff-primed” cells generated higher forces than those trained on soft matrices, which allowed them to more effectively remodel surrounding collagen fibers, regardless of fiber density, and spur invasion into new environments.
“Our bodies are largely composed of collagen, which is a very versatile material that cells can deform and interact with in various ways,” Almeida said. “That makes this question of how previous environments affect future environments really complex. Looking at this 3D collagen matrix lets us see cross-environmental effects, including how normal or healthy cells are impacted by primed cells.”
Based on the fundamental physics of collagen deformation and cell movement combined with Almeida’s experimental results, Mathur expanded the existing model of cell migration to account for how cells’ mechanical memory is transferred onto the matrix through collagen alignment and tension. By remodeling their surrounding matrix, cells lower the environmental resistance they encounter, which eases further cell migration. The advantage gained by primed cells then persists over time and is especially powerful in challenging environments.
“Our model includes key aspects of how cells are modifying their environment, how properties from the first environment allow modification of a secondary environment, and how those features continue from one environment to the next,” Mathur said.
“We started by thinking about cancer. When tumors develop, the tissue becomes stiffer, and we know cells migrate faster in a stiff environment. But outside the tumor, there’s soft, healthy tissue, so why do tumor cells continue to migrate fast during metastasis? It’s counterintuitive unless the cells are remembering their past stiff environment.”
By understanding exactly how mechanical memories are stored by cells and shared across environments, the team can make predictions about future cell migration and provide insight into the progression of diseases, such as cancer metastasis and fibrosis development, as well as normal developmental and aging processes. Their plans for future work include further exploration of cell priming, how the extracellular matrix influences cellular gene profiles, and possible interventions to slow or stop undesirable invasions, including targeted therapeutics for cancer treatment.
More information:
José A. Almeida et al, Mechanically primed cells transfer memory to fibrous matrices for invasion across environments of distinct stiffness and dimensionality, Molecular Biology of the Cell (2023). DOI: 10.1091/mbc.E22-10-0469
Provided by
Washington University in St. Louis
Citation:
Environmental memory propels collective cell migration, shows study (2023, May 19)