CRISPR-Cas systems help to protect bacteria from viruses. Several different types of CRISPR-Cas defense systems are found in bacteria, which differ in their composition and functions. Among them, the most studied proteins today are Cas9 and Cas12, also known as DNA or “gene scissors,” which have revolutionized the field of genome editing, enabling scientists to edit genomes and correct disease-causing mutations precisely.
Researchers from the Institute of Biotechnology at the Life Sciences Center of Vilnius University—Dalia Smalakytė, Audronė Rukšėnaitė, Dr. Giedrius Sasnauskas, Dr. Giedrė Tamulaitienė, and Dr. Gintautas Tamulaitis—have revealed the structure of the CRISPR-Cas protein scissors found in bacteria and provided mechanistic details on how they function. The results of their study have been published in Molecular Cell.
A team of researchers led by Dr. Tamulaitis is studying the bacterial defense system CRISPR-Cas10, which acts as a sensor. When a virus attacks the bacterium, it sends a “message” by synthesizing unique signal molecules called cyclic oligoadenylates.
These signaling molecules are recognized by different effectors, i.e. accessory proteins in the system that enhance the bacterial defense against viruses. A recent computational analysis predicted that CRISPR-Cas10 effectors may have diverse enzymatic activities, allowing bacteria to defend themselves against viruses in multiple ways.
“The discovery of cyclic oligoadenylates and the understanding of the mechanism of CRISPR-Cas10 have sparked great scientific interest and a breakthrough in signaling pathway research. Recently, a similar protection principle has been identified in other bacterial defense systems: CBASS, Pycsar, and Thoeris. In this study, we investigated the tripartite CalpL-CalpT-CalpS effector which is activated by CRISPR-Cas10 signaling molecules and we explained how this complex system works and how it is regulated,” explains Dr. Tamulaitis.
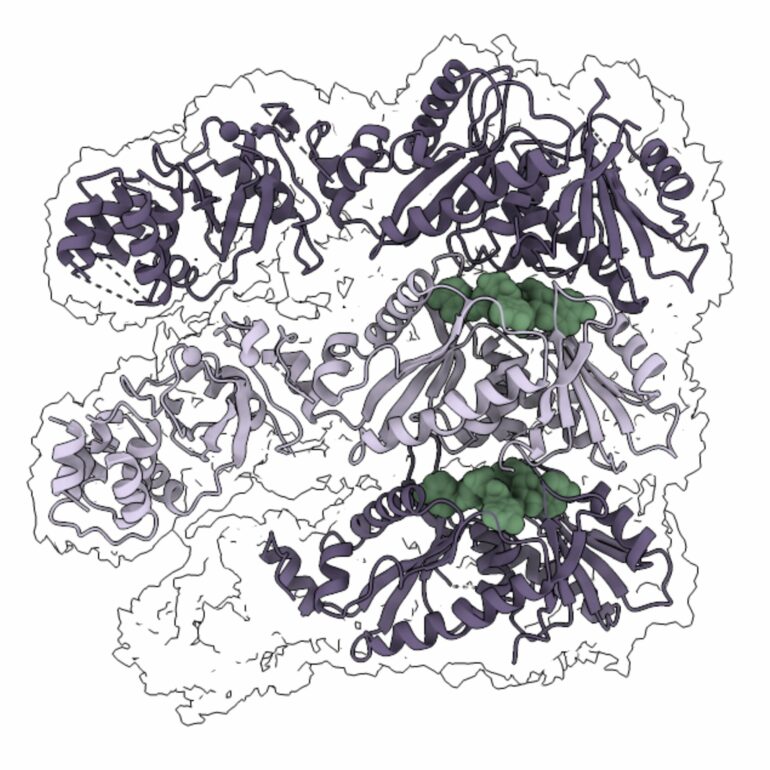
The CalpL-CalpT-CalpS effector consists of three key proteins: CalpL, which acts as a signal-recognizing protein scissors; CalpS, a protein that regulates gene expression; and CalpT, an inhibitor of the CalpS protein. The researchers used a combination of biochemical, biophysical, bacterial survivability assays, and cryogenic electron microscopy (cryo-EM) to study this system. They found that when CalpL binds a molecule that signals a viral infection, it forms a polymeric filament of variable composition.
The filament structure allows the CalpT-CalpS heterodimer to attach, positioning the active center of the scissors CalpL near the inhibitor CalpT and enabling it to cleave it. Once CalpT has been cloven, CalpS is released from the heterodimer and can regulate gene expression to protect the bacterium from viral infection.
One of the authors, Dalia Smalakytė, points out that the activity of the CRISPR-Cas protein scissors is tightly regulated in time. The protein scissors have an internal timer mechanism that is activated upon the binding of signaling molecules and filament formation. This mechanism is unique compared to other similar signal-sensing effector proteins.
The newly discovered mechanism of the CRISPR-Cas10 system illustrates the complexity of the bacterial defense system. These studies pave the way for the practical application of the regulated CRISPR-Cas protein scissors as a molecular indicator of infection.
More information:
Dalia Smalakyte et al, Filament formation activates protease and ring nuclease activities of CRISPR Lon-SAVED, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.09.002
Provided by
Vilnius University
Citation:
Filament structure found to activate and regulate CRISPR-Cas ‘protein scissors’ (2024, October 2)