A team of WEHI researchers has for the first time visualized a human cell death complex linked to autoimmune and inflammatory conditions, such as inflammatory bowel disease, and injuries associated with excessive cell death.
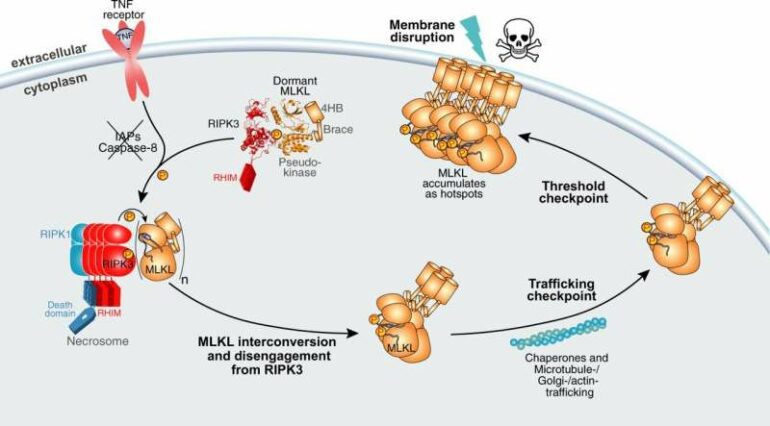
Using the Australian Synchrotron, the team solved the structure of the human cell death proteins MLKL and RIPK3 bound to each other, as well as human RIPK3 alone. When RIPK3 activates MLKL, it triggers a type of inflammatory cell death called necroptosis that kills the cell and alerts the immune system that it is under attack. However, when uncontrolled, necroptosis has been linked to human inflammatory diseases.
The findings will help scientists discover drugs that can target and suppress cell death by necroptosis, which could lead to new treatments for a range of autoimmune and inflammatory diseases including inflammatory bowel disease, renal injury and diabetes.
The research, published in Nature Communications, was led by WEHI researchers Yanxiang Meng, Dr. Katherine Davies, Associate Professor Peter Czabotar and Associate Professor James Murphy. The discovery is the latest in an almost 15-year-long journey to understand necroptosis for treating disease.
‘The cell death pathway’
Necroptosis is a type of inflammatory cell death process that helps protect the body against infection. Most often triggered when a cell is infected by a virus or bacteria, the cell is instructed to die and send inflammatory signals to warn the immune system of foreign invaders. However, when necroptosis is uncontrolled or excessive, the inflammatory response can trigger disease.
Ph.D. student Yanxiang Meng said that MLKL and RIPK3 are bound in an inert state in all cells of the body, waiting to be activated.
“MLKL and RIPK3 form an inert complex, with RIPK3 ‘holding’ MLKL in an inactive state to prevent necroptotic cell death,” he said.
“When the cell is infected, RIPK3 chemically modifies MLKL then detaches, giving it a ‘licence to kill’ the infected or damaged cell for the greater good,” he said.
He said the Australian Synchrotron and Collaborative Crystallisation Centre (C3) facility at CSIRO were crucial to visualize the structure of human forms of RIPK3 bound to human MLKL for the first time.
“The necroptotic cell death proteins are conserved across different organisms, however there are differences between the proteins’ structures in different animals and how they bind to each other.
“We showed that the human versions of these proteins bind differently to what we have seen in other species. This is something the scientific community has been waiting many years for.”
New targets for drug discovery
Dr. Davies said the team hoped this structural information would, in the future, lead to new treatment options for patients suffering from diseases linked to excessive necroptosis.
“We now have a picture of how two key proteins in this death pathway are maintained in their dormant state. It would be interesting to know how this is regulated and leads to disease and whether this could be targeted with small molecule drugs,” she said.
More information:
Yanxiang Meng et al, Human RIPK3 maintains MLKL in an inactive conformation prior to cell death by necroptosis, Nature Communications (2021). DOI: 10.1038/s41467-021-27032-x
Provided by
Walter and Eliza Hall Institute of Medical Research
Citation:
First structure of human protein complex with ‘licence to kill’ (2021, December 10)