Researchers have unraveled a key genetic mechanism behind the way pathogens infect crops, leading to new strategies for breeding resistant crop varieties against other pathogens carrying the same genetic mechanism.
Led by researchers from the Center for Crop and Disease Management (CCDM)—a national center co-supported by GRDC and Curtin University—along with collaborators from the Curtin Health Innovation Research Institute, CSIRO, the University of Nottingham (UK) and INRAe (France), the team identified and validated the function of a specific DNA sequence linked to genes that cause damage on wheat.
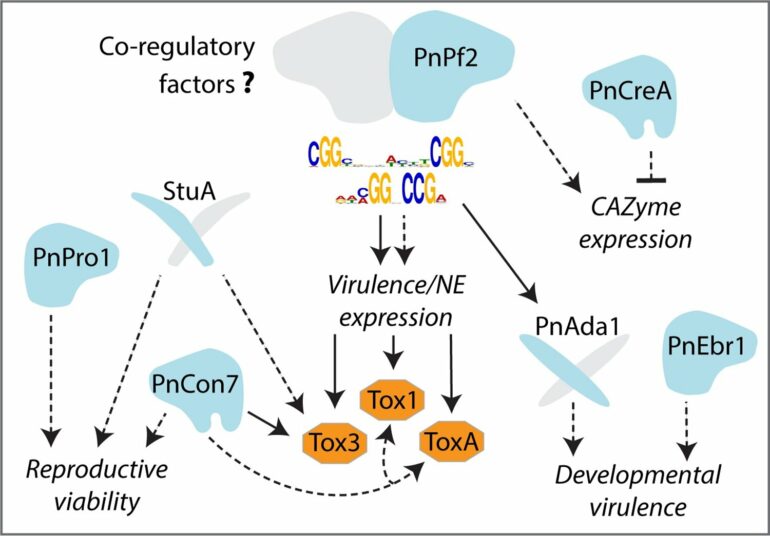
By studying the genetic mechanism in Parastagonospora nodorum, the fungus that causes septoria nodorum blotch (SNB) of wheat, the research team were able to confirm that a transcription factor called Pf2 binds to a specific DNA consensus sequence. By doing this, Pf2 activates adjacent genes to produce necrotrophic effectors—molecules responsible for inducing damage on wheat.
CCDM’s former Ph.D. student Dr. Evan John and Associate Professor Kar-Chun Tan, along with their research team, are hoping to transfer this knowledge to other diseases to improve the identification of necrotrophic effectors and other virulence-associated genes. These findings are published in the journal PLOS Pathogens.
“This discovery of the DNA consensus sequence is a big deal for disease resistance breeding research, as it means we now know how the pathogen’s effectors are activated to attack a plant,” Dr. John said.
“What’s exciting about this research, is that it can be used as a regulatory model, because the same Pf2 transcription factor is found in other fungal pathogens that cause diseases such as yellow spot of wheat, blackleg and black spot of canola. Based on our current knowledge, it looks like Pf2 is operating by the same mechanism there.”
Associate Professor Tan said for many years, researchers have been searching for effectors in pathogen genomes, however narrowing them down to a short candidate list can be difficult.
“Now, knowing the genetic code of the DNA sequence targeted by Pf2, we can narrow down potential effector genes that are associated with the specific DNA consensus sequence, and prioritize these genes for effector discovery,” Associate Professor Tan said.
“Finding effectors is a big win, because it means we can then find the corresponding susceptible gene within the crop, and help breeders by using effector-assisted selection on crops, providing growers with varieties with improved disease resistance.”

The CCDM research team, including (left to right) Dr Callum Verdonk, Leon Lenzo, Associate Professor Kar-Chun Tan, Dr Evan John, Dr Jordi Muria Gonzalez and Shota Morikawa. © Grains Research & Development Corporation
CCDM Director Professor Mark Gibberd said he was proud of how far this CCDM research team had come in reaching such an important scientific outcome that will lead to better varieties for growers.
“This discovery was eight years in the making. A few years ago, the team had discovered the transcription factor and knew that it regulated effectors, but were unsure how it regulated them,” Professor Gibberd said.
“By persisting through countless challenges, they have gotten to the bottom of the scientific mystery, and have reached a conclusion that will help improve disease resistance not only in wheat, but potentially against canola diseases too.
“This research is an example of CCDM’s ability to work deeply and collaboratively on blue-sky research to ensure Australian agriculture is a global leader in research and innovation when it comes to grain production.”
More information:
Evan John et al, Regulatory insight for a Zn2Cys6 transcription factor controlling effector-mediated virulence in a fungal pathogen of wheat, PLOS Pathogens (2024). DOI: 10.1371/journal.ppat.1012536
Provided by
Grains Research & Development Corporation
Citation:
Genetic mechanism unlocks a key secret behind disease infection in crops (2024, October 10)