Biological cells have many vital functions in the organism. For example, they produce proteins, carbohydrates and fats. But they are also responsible for detoxifying harmful molecules and transmitting signals and immune defense steps. A so-called redox potential is required to drive these processes. It depends on the ratio of NADPH (nicotinamide adenine dinucleotide phosphate in negatively charged, “reduced” form) to its oxidized form NADP⁺.
A team led by plant biotechnologist Prof Markus Schwarzländer from the University of Münster and biochemist Prof Bruce Morgan from Saarland University has now developed new biosensors with which the ratio of NADPH to NADP⁺ can be measured in living cells in real time for the first time. The team’s observations provide new insights into the evolution of the most important protective function of cells, cellular detoxification. The study is published in Nature Communications.
NADP is involved in many reactions in the cell in which electrons are transferred between different substances. “You can visualize the ratio of NADPH to NADP⁺ like the charge of a rechargeable battery,” explains Schwarzländer. However, all biological cells have many different batteries, which also have different charges in different areas of the cells.
“Until now, only some of these batteries could be read out, or the cells had to be destroyed to do so, which falsifies the measurements,” explains Ph.D. student Jan-Ole Niemeier. The scientists have now developed a family of biosensors that are genetically encoded and thus produced by the cells themselves and transported to the right place in the cell. These biosensors can be read by light or fluorescence so that they can be used non-destructively in living cells and tissues.

Prof Bruce Morgan, PhD student Anika Diederich, PhD student Jan-Ole Niemeier and Prof Markus Schwarzländer (from left) in the laboratory with the recombinant sensor protein (in pink) isolated from bacterial cells. © Markus Schwarzländer
For the new sensors, the scientists used genetic engineering methods to modify a previously developed fluorescent molecule, which contains parts of a luminescent jellyfish protein, in such a way that it specifically recognizes NADPH and NADP⁺.
Among other things, the team discovered that the “NADP charge” is very robust and is recharged particularly efficiently by cellular metabolism when required. They also observed “NADP charge cycles,” i.e., oscillations of the cell battery, during cell division, and the influence of photosynthesis and the availability of oxygen on the NADP battery.
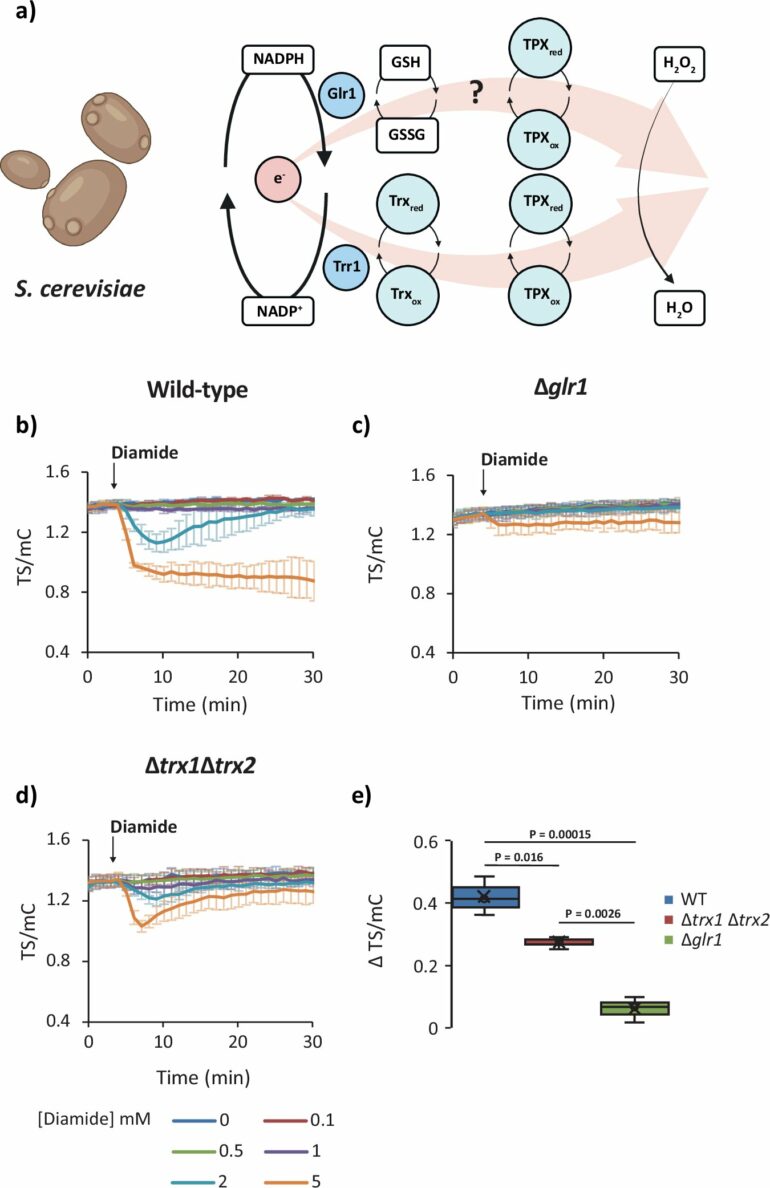
Another important finding was that the detoxification of reactive oxygen species—such as hydrogen peroxide—takes place primarily via the glutathione present in the cells (a tripeptide that is present in the cell in comparatively high concentrations), regardless of whether in yeast, plant or mammalian cells. “This finding challenges the prevailing view that the so-called thioredoxin detoxification pathway is particularly important for the defense against oxidative stress,” emphasizes Bruce Morgan.
Other groups involved in the project include the team of cell biologist Prof Carsten Grashoff, also from the University of Münster, as well as groups from the Universities of Cologne and Brussels.
More information:
Marie Scherschel et al, A family of NADPH/NADP+ biosensors reveals in vivo dynamics of central redox metabolism across eukaryotes, Nature Communications (2024). DOI: 10.1038/s41467-024-55302-x
Provided by
University of Münster
Citation:
Genetically encoded biosensors measure living cells’ charge in real time (2024, December 20)