Trematodes, also known as flukes, are a class of parasitic flatworms with intricate lifecycles. This makes them interesting to scientists, but they are also significant to both human health and wildlife conservation.
Trematodes can cause infection in humans when people eat food the flatworms have contaminated, including raw fish, crustaceans and vegetables. Though this is not a major issue in the United States, the World Health Organization estimates foodborne trematode infection causes the loss of more than 2 million years of life to disability and death worldwide every year, with different species causing cancer, liver cirrhosis and cerebral hemorrhage in extreme cases.
Some trematodes also infest amphibians, contributing to the 3.79% yearly average decline in their overall population. Trematodes such as Ribeiroia ondatrae can play a role in this decline, causing serious malformations in 80% to 90% of frogs in some regions of North America, according to Dana Calhoun, a University of Colorado Boulder senior research associate in the Department of Ecology and Environmental Biology.
Her recent research shows that different species of trematodes have different distributions throughout certain amphibians’ bodies, and that parasites went to different places in the bodies of the animals studied. These findings may help scientists better understand the mechanisms of infection.
To understand trematodes more completely, Calhoun, along with Pieter Johnson, a CU Boulder professor of distinction in the Department of Ecology and Evolutionary Biology, and researchers Jamie Curtis and Clara Hassan used heatmaps to characterize the distribution of several trematode species in Pacific tree frogs and two species of newts. Their findings were published in the Journal of Helminthology.
The complex lifecycle of digenetic trematodes
Some parasites infect only hosts of a single species while others can infect thousands. One of the attributes that makes digenetic trematodes (those belonging to the subclass Digenea) so special, Calhoun says, is their standard life cycle requires three hosts to complete.
“The main host is called the definitive host,” Calhoun explains. “That could be a mammal, a larger frog, a fish or a bird. In an aquatic system, that host would defecate into the water.” The eggs inside the fecal material hatch and the trematodes try to make their way into their first intermediate hosts. “In the freshwater system, it’s usually a snail,” Calhoun says.
Once the trematodes are in the snails, the hosts will survive, but they are castrated—unable to reproduce. According to Calhoun, this is because “the parasite takes over the gonad area and uses all the resources of the snail to reproduce asexually. This allows the parasite to release a couple of thousand free-living cercariae from the snail daily.”
Cercariae are the larval form of trematodes, and given that they are sperm sized, Calhoun says, “there need to be a lot of them if they’re going to get where they need to go within 24 hours,” especially given that they are often preyed upon at that stage.
After that, the parasites will try to enter the second intermediary host, which in this case is generally an amphibian but could also be a fish, invertebrate, or anything that is likely to be eaten by the final, or definitive, host, Calhoun says.
This stage is particularly interesting to parasitologists because parasites sometimes alter their hosts to make transmission to the definitive host more likely. For example, the species Ribeiroia ondatrae is able to make frogs grow extra legs, as mentioned in Calhoun’s study.
The trematodes are passed from the secondary intermediate host to the definitive host when the definitive host eats the secondary host. Amphibians with limb deformities caused by trematodes of the genus Ribeiroia are hypothesized to be caught and eaten by definitive hosts more often, according to Calhoun. Parasites are in a somewhat awkward position at this point, given that they don’t want to cause the death of their hosts but do want to be eaten to complete their final stage of their life cycle.
Trematodes also can sometimes have a fourth host, called the paratenic host. These hosts allow parasites to survive during times when definitive hosts are not accessible, Calhoun says.
“It’s a holdover, since the frog will eventually leave the water and that will make it hard for birds to eat it,” she adds. The parasite’s development is paused while it is in a paratenic host.
“Simplified lifecycles are much easier,” Calhoun notes, yet the trematode lifecycle requires many stages of the animal’s life to play out in a particular way. “It seems like there are a lot of ways for this kind of lifecycle to be interrupted, but it’s still so common. Trematodes are all over the world, and to me, that’s what makes them very special.”
Different body parts, different parasites
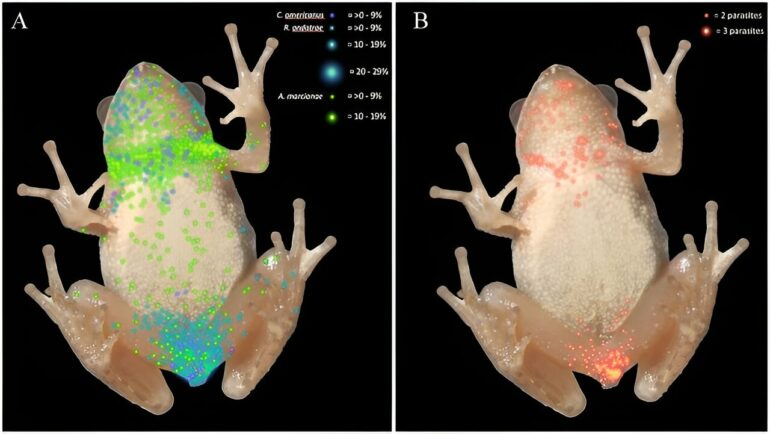
To produce the final heatmaps showing where the parasites gathered in the amphibians’ bodies, the researchers dissected the frogs and newts and recorded the number and type of parasites in each cell of a grid of the animals’ bodies.
This revealed that different species of parasite (Alaria marcianae, Cephalogonimus americanus, several species of Echinostoma and Ribeiroia ondatrae) had different distributions, and that parasites went to different places in the bodies of the newts than they did in the frogs.
In the frogs, most of the Echinostoma were found in the kidneys, most of the Ribeiroia were found in the front of the body, most of the Alaria were found in the back of the body (particularly around the neck), and Cephalogonimus trematodes were spread roughly evenly between the front and back. Both Ribeiroia and Cephalogonimus were concentrated in specialized regions and can therefore be considered specialist parasites.
“Specialists usually use a certain area or microhabitat within the host, or they could even specialize in the host itself,” Calhoun says. For example, one of the parasites she studies, Scaphanocephalus, uses osprey as its definitive host, whereas other trematodes can live in all taxa of birds that eat fish. Because of that, there is a risk that the intermediate host will be eaten by a bird other than an osprey, disrupting the parasite’s lifecycle.
There also is some risk of the host species going extinct, which would force the parasite to find a different host. However, Calhoun explains, specialists are adapted to their definitive host, making it less likely that the host will successfully remove them or kill them with its immune system. Moreover, specializing in one animal or area within an animal can reduce competition.
The study also showed that Ribeiroia clustered in different places in newts than in frogs. Ribeiroia were the only parasites found in the newts collected for the study, and they were concentrated in the tissues near the front of the body rather than those nearer to the back.
Calhoun says this may be because they enter newts’ bodies differently: “One of the characteristics that’s different between the hosts in the study are that newts are carnivores, so it may be that they are trying to eat the parasites when they are in the water system and therefore getting infected that way.”
The benefits of using heatmaps
Coinfection is the simultaneous infection of a host by multiple pathogen species. According to Calhoun, researchers have seen coinfection by multiple trematodes through wild animal dissection. This study shows that coinfecting parasites often occupy the same spaces, or microhabitats, in hosts.
“In one of our figures,” Calhoun says, “we show that both parasites would be in the same tiny grid cell very commonly. You have this whole dermis of the animal, but they all go to the same area. Heatmaps allowed us to see it, but now we need to investigate why.”
Calhoun says that heatmaps could be generally useful for specifying the positions of parasites within hosts.
“If I dissect something and I find parasite A in the head and parasite B in the limb,” she explains, “it’s so broad to just say ‘in the head’ or ‘in the limb’ because those areas are so large compared to the parasites. What heatmaps show is that they are in the same 1-by-1 cells on the heatmap grid, basically stacked on top of each other. It shows that there are these coinfection areas in the host that are attractive to parasites compared to other locations. It’s important for researchers to study these microhabitats, especially why multiple parasites use the same microhabitat.”
While some parasites congregate in the same spaces, others do not. For example, Calhoun says that Echinostoma is, for the most part, the only species of parasite found in the kidneys of the amphibians from the study. This suggests that they are uniquely adapted to enter this region of the body.
Heatmaps also helped the researchers explain why frogs infected with Ribeiroia from one region that researchers visited were not malformed as often as frogs from another region.
“In the (San Francisco) Bay Area, you would see (frogs with) six or seven legs, with high loads of Ribeiroia, but we weren’t seeing that same phenomenon in the high elevation areas of California, when load of Ribeiroia was sometimes double,” Calhoun says.
“To explore this trend, we examined the heatmaps of Ribeiroia infections in both areas and found that, for some reason, Ribeiroia in high elevation were going to the head and (jaw) region, not to the hindlimbs. It could be related to the timing. It’s much colder in mountains, and it doesn’t get warm until August in this area, which is when the frogs start to metamorphosize. Therefore, the lack of malformations could be related to missed timing of parasite release and frog development.”
Another method involves using florescent labeling. In a laboratory setting, Calhoun explains, labeling the parasites with color prior to their entering the frogs would allow researchers to track how they move, what the consequences of that movement are and whether their location changes over time.
“And if you use dyes like this,” she says, “you could explore single infections in an animal with Echinostoma, Cephalogonimus, or Alaria, and then add in another parasite and see if the distribution changes. Others have demonstrated that with multiple parasites, as you add taxa, the parasites move to different areas. But how does that occur? Where do they go? What if we introduced them at the same time? Using this method combined with heatmaps, a researcher could investigate the mechanism of infections.”
More information:
Dana M. Calhoun et al, Putting infection on the map: Using heatmaps to characterise within- and between-host distributions of trematode metacercariae, Journal of Helminthology (2023). DOI: 10.1017/S0022149X2300069X
Provided by
University of Colorado at Boulder
Citation:
Heatmaps show trematodes congregate in certain parts of amphibians’ bodies, often to dire physical consequences (2024, May 24)