The evolution of pathogens has received attention in a wide range of scientific fields, such as epidemiology, demography, and evolutionary ecology. Understanding pathogen evolution is particularly urgent for rapidly evolving pathogens, such as SARS-CoV-2, which has spread globally since 2019.
Classical evolutionary theory states that virulence evolves to maximize a pathogen’s basic reproduction ratio, i.e., the average number of secondary infections caused by one infected host. This approach provides insights into how pathogen virulence evolves under tradeoffs with other epidemiological parameters such as the rates of infection and recovery. Over time, the classical theory has been extended to a variety of ecological and epidemiological contexts.
Despite these advancements, most models continue to assume homogeneous host populations, thereby neglecting the impacts of all environmental heterogeneity.
In reality, however, ecosystems often represent interconnected local populations that experience different local conditions, as described by ecological metapopulation theory. Moreover, the movement patterns of host individuals connecting these local populations also tend to be heterogeneous, and such imbalances in movement among local populations creates a “source-sink” structure, with some populations (sources) creating a net outflow and other populations (sinks) receiving a net inflow.
To address this gap, researchers have developed an evo-eco-epidemiological metapopulation model to investigate how heterogeneity in local environments and movement networks influences the evolution of pathogen virulence and infectivity, providing new insights into the evolution of pathogens in diverse ecological contexts. The paper is published in the journal Proceedings of the National Academy of Sciences.
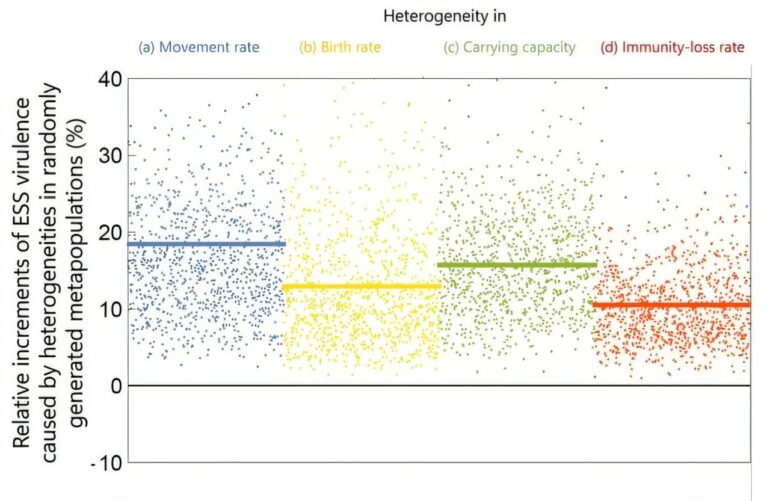
The analyses reveal that pathogen virulence consistently increases in metapopulations with heterogeneous local conditions. Even with modest heterogeneities (10% variation) in host movement rates, birth rates, carrying capacities, or immunity-loss rates, the evolved virulence is, on average, 20% higher—and up to 40% higher—compared to homogeneous metapopulations.
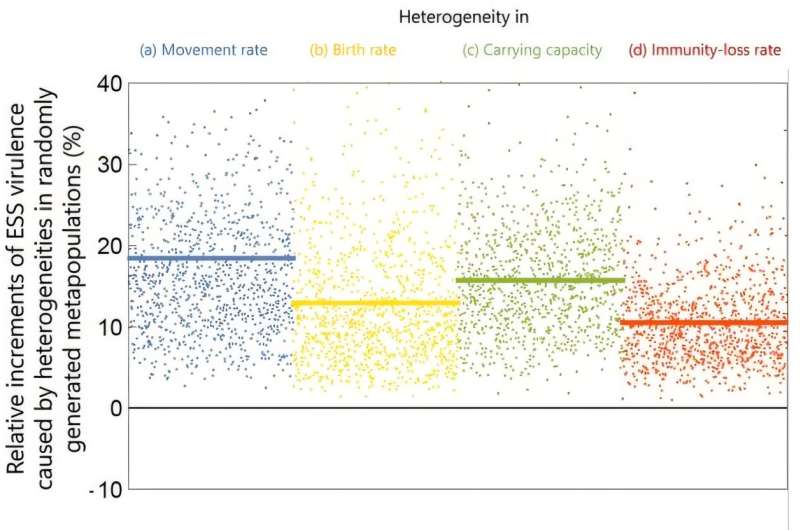
Evolved virulence is consistently higher in heterogeneous metapopulations compared to homogeneous metapopulations. Each point represents the evolved virulence in a metapopulation with randomly generated local environments that are heterogenous in terms of movement rates, birth rates, carrying capacities, or immunity-loss rates. The horizontal lines indicate the resultant average increases in the evolved virulence. © SOKENDAI, AIST, OIST
Why does environmental heterogeneity drive the evolution of higher pathogen virulence and infectiousness? Through perturbation-expansion methods and evolutionary dynamical analysis, the researchers have uncovered the underlying general mechanism.
Heterogeneity creates variation across local populations in the availability of uninfected hosts, which serve as resources for pathogens in their quest to infect new hosts, and it is ultimately this variation that promotes the evolution of more virulent pathogens. For instance, in local populations with higher carrying capacity, host density is elevated, creating a “richer” environment that favors aggressive pathogens. These pathogens cause more severe symptoms, are more highly infectious, and exploit their hosts more rapidly.
In contrast, in local populations with lower carrying capacity, host density is diminished, which is limiting the availability of uninfected hosts. Here, milder pathogens are favored evolutionarily, as they can better persist under such resource-scarce conditions.
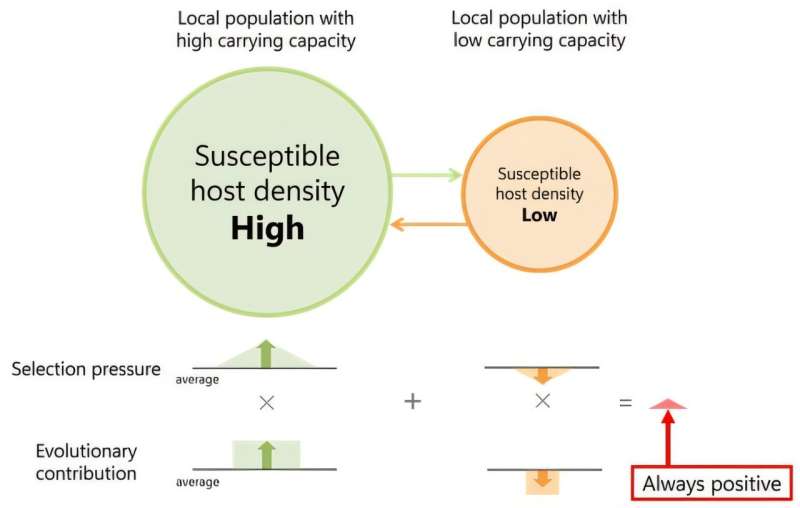
Heterogeneities in local environments create heterogeneity in the densities of local host populations. The left population is “resource-rich” for pathogens, as it offers a higher density of susceptible hosts, while the right population is “resource-scarce”, as it offers a lower density of susceptible hosts. For higher susceptible-host densities, more virulent pathogens are favored, while for lower susceptible-densities, milder pathogens are favored. Crucially, the net effect of these selection pressures across the metapopulation is not balanced, as the resource-rich population makes a more significant evolutionary contribution than the resource-scarce population, causing evolution to drive up pathogen virulence and infectiousness. © SOKENDAI, AIST, OIST
However, these opposing local evolutionary trends do not balance out in the evolution of pathogens across a metapopulation. Pathogens in resource-rich populations produce more infections and contribute more significantly to the gene pool of the metapopulation, and thus have higher evolutionary importance.
This results in an evolutionary bias toward higher virulence, as the selection for aggressive pathogens outweighs the selection for milder ones. Consequently, environmental heterogeneity consistently drives the evolution of higher pathogen virulence across metapopulations.
This study establishes a foundation for understanding pathogen evolution in heterogeneous metapopulations, paving the way for various extensions, such as continuously spatially structured host populations, distributed public-health interventions, and diverse pathogen-transmission modes, including zoonoses and vector-borne infections.
More information:
Masato Sato et al, Metapopulation heterogeneities in host mobility, productivity, and immunocompetency always increase virulence and infectiousness, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2309272121
Provided by
The Graduate University for Advanced Studies, SOKENDAI
Citation:
Heterogeneous host populations drive evolution of more virulent pathogens, modeling study shows (2024, December 20)