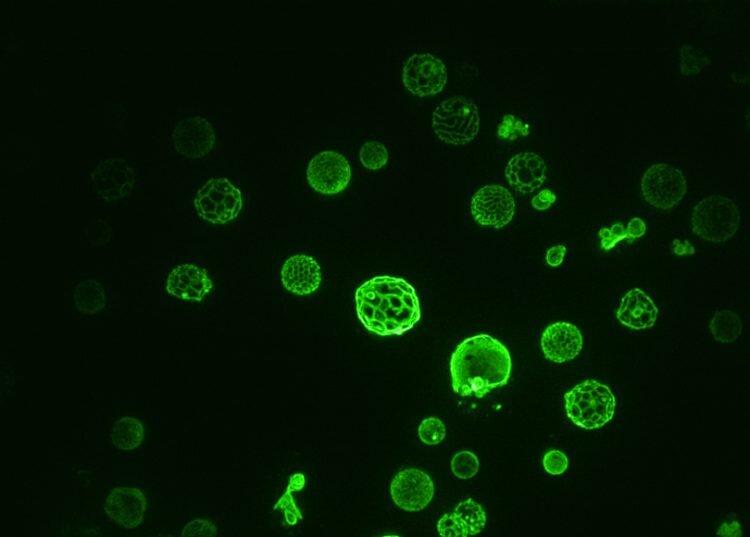
Membranes are crucial to our cells. Every cell in your body is enclosed by one. And each of those cells contains specialized compartments, or organelles, which are also enclosed by membranes.
Membranes help cells carry out tasks like breaking down food for energy, building and dismantling proteins, keeping track of environmental conditions, sending signals and deciding when to divide.
Biologists have long struggled to understand precisely how membranes accomplish these different types of jobs. The primary components of membranes—large, fat-like molecules called lipids and compact molecules like cholesterol—make great barriers. In all but a few cases, it’s unclear how those molecules help proteins within membranes do their jobs.
In a paper published Jan. 25 in the Proceedings of the National Academy of Sciences, a team at the University of Washington looked at phase separation in budding yeast—the same single-celled fungus of baking and brewing fame—and reports that living yeast cells can actively regulate a process called phase separation in one of their membranes. During phase separation, the membrane remains intact but partitions into multiple, distinct zones or domains that segregate lipids and proteins. The new findings show for the first time that in response to environmental conditions, yeast cells precisely regulate the temperature at which their membrane undergoes phase separation. The team behind this discovery suggests that phase separation is likely a “switch” mechanism that these cells use to govern the types of work that membranes do and the signals they send.
“Previous work showed that these domains can be seen in the membranes of living yeast cells,” said lead author Chantelle LeveilIe, a UW doctoral student in chemistry. “We asked: If it’s important for a cell to have these domains, then if we change the cell’s environment—by growing them at different temperatures—would the cell ‘care’ and devote energy to maintaining phase separation in its membranes? The clear answer is yes, it does.”
Past research has shown that when sugar is plentiful, the yeast cell’s vacuole—an important organelle for storage and signaling—grows large and its membrane appears uniform under a microscope. But when food supplies dwindle, the vacuole undergoes phase separation, with many round zones appearing in the organelle’s membrane.
In this new study, Leveille and her co-authors—UW chemistry professor Sarah Keller, UW biochemistry professor Alexey Merz and Caitlin Cornell, previously a UW doctoral student in chemistry—sought to understand whether yeast can actively regulate phase separation. Leveille grew yeast at their typical laboratory temperature of 86 F with plenty of food. After the food dwindled, the yeast cell vacuole membranes underwent phase separation, as expected. When Leveille briefly raised the temperature in the yeast’s environment by about 25 degrees Fahrenheit, the domains disappeared. Then Leveille grew yeast at a cooler temperature—77 F instead of the normal 86 F—and discovered that the domains disappeared about 25 degrees above this new temperature. When she grew yeast in still colder conditions, at 68 F, phase separation yet again disappeared about 25 degrees higher than their growth temperature.
These experiments showed that the yeast cells always maintained phase separation in the vacuole membrane until the temperature rose about 25 degrees above their growth temperature.
“We think this is a clear sign that yeast cells are engineering the vacuole membrane in different environmental conditions to maintain this consistent state of phase separation,” said Leveille.
Phase separation in the vacuole membrane likely serves an important purpose in yeast, she added.
“This result suggests that membrane phase separation for yeast is likely a two-way door,” said Leveille. “For example, if the cells ever found food again, they would want to go back to their original state. Yeast do not want to get too far away from the transition.”
Future research could identify other membrane components that affect the vacuole membrane’s ability to phase separate, as well as the consequences of its phase separation. Biologists have known that when the domains appear in the yeast vacuole’s membrane, the cell stops dividing. These two events may be linked because the yeast vacuole’s membrane contains two complexes of proteins that are important for cell division. When the complexes are far apart, cell division stops.
“Phase separation in the vacuole occurs right when the yeast cell needs to stop dividing because its food supply has run out,” said Merz. “One idea is that phase separation is the mechanism that the yeast cell ‘uses’ to separate these two protein complexes and stop cell division.”
In cells from yeast to humans, protein complexes embedded in membranes affect cell behavior. If additional research shows that phase separation in the yeast vacuole regulates cell division, it would likely be the first rigorous example of cell regulation through this once-overlooked property of membranes.
“Phase separation could be a common, reversible mechanism to modulate many, many types of cellular properties,” said Keller.
Cornell is now a postdoctoral researcher at the University of California, Berkeley.
More information:
Chantelle L. Leveille et al, Yeast cells actively tune their membranes to phase separate at temperatures that scale with growth temperatures, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2116007119
Provided by
University of Washington
Citation:
Hungry yeast are tiny, living thermometers (2022, January 25)