An unprecedented glimpse of the human embryo at an early stage of development has provided critical clues on how undifferentiated cells become the specialized ones of which we are made, researchers reported on Wednesday.
As an embryo begins to form, it is composed of stem cells with the potential to become any part of the body, from brain matter to bone tissue.
Human stem cells begin to take on these specific roles during a process called gastrulation that occurs starting in the third week after fertilization.
Up to now, that process has been mostly a black box, inaccessible to direct viewing.
Ethics rules mean that lab-grown stem cells can only be grown artificially for two weeks. It is also impossible to observe gastrulation during pregnancy.
The new findings, published in Nature, provide direct data of how the transformation of stems cells takes place.
Experts not involved in the study hailed it as a “landmark” and a “Rosetta Stone” for future research in developmental biology.
Up to now, scientists have relied on samples from mice and non-human primates to better understand gastrulation, but the degree of similarity to the process in humans was always in doubt.
The data presented on Wednesday provides a basis for measuring how useful experiments on other mammals have been and will be in the future.
No neurons
Human cells contain all of a person’s genetic material, but gastrulation marks the initial stage when certain genes get switched on.
It is the first step in determining whether a cell will become part of our blood, for example, or a brain cell.
“You have a kind of explosion of cell diversity,” study author Shankar Srinivas of Oxford University told journalists in an online press conference, describing the process as “beautiful”.
The cells that make up an embryo at this stage begin clustering into specific regions.
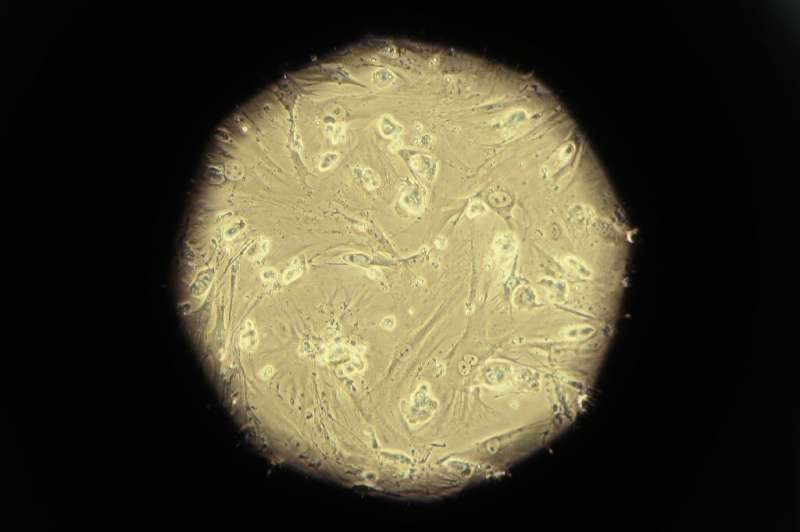
Ethics rules mean that lab-grown stem cells can only be grown artificially for two weeks.
Srinivas’s team dissected the sample of a donated human embryo and then used a process called single-cell RNA sequencing to determine which genes were active in each of the more than 1,000 individual cells.
The resulting map shows which cells had been activated to take on certain roles, and where they were located in the weeks-old embryo.
Matching the findings with observations of mouse embryos, researchers found more similarities than differences.
“A mouse is actually a very good model of a human,” Srinivas said.
But there were key differences, such as the presence of proto-blood cells in humans much earlier than in mice.
And scientists also noted an important absence: while mice embryos at this stage would have begun to develop a nervous system, there was no such material present in the human sample.
That finding could help raise the 14-day limit on culturing embryos for study, which was set to completely rule out study on an embryo with even the beginnings of a nervous system.
Rare sample
Both scientists involved with the study and outside observers noted the exceptional rarity of the sample, which was sourced from the Human Developmental Biology Resource in the UK.
“Most people wouldn’t even know they were pregnant in the 16 days after fertilization,” said Srinivas, “But this person did, had the termination and generously donated the sample.”
Srinivas said before that his lab spent five years on a waiting list—and that a rules change in how terminated embryos are collected would probably mean a much longer wait in the future.
His team conducted genomic tests and physical examinations to determine the sample was a good representation of normal human development.
But they also said it would be ideal to have more such samples to compare, and that a change in rules may be necessary to allow that to happen.
“It is a landmark paper, upon which many will base their future findings,” said geneticist Darren Griffin of the University of Kent.
Stem cell biologist Harry Leitch of the Imperial College London called it “an invaluable resource” that will “facilitate further advances in stem cell biology and regenerative medicine”.
More information:
Richard C. V. Tyser et al, Single-cell transcriptomic characterization of a gastrulating human embryo, Nature (2021). DOI: 10.1038/s41586-021-04158-y
2021 AFP
Citation:
‘Landmark’ study probes crucial phase of embryo development (2021, November 17)