New findings published in Molecular Cell provide details about the hidden organization of the cytoplasm—the soup of liquid, organelles, proteins, and other molecules inside a cell. The research shows it makes a big difference where in that cellular broth, messenger RNA (mRNA) gets translated into proteins.
“You know the old real estate saying, ‘location, location, location.’ It turns out it applies to how proteins get made inside of cells, too,” says Dr. Mayr, a molecular and cell biologist at the Sloan Kettering Institute, a hub for basic and translational research within MSK. “If it’s translated over here, you get twice as much protein as if it’s translated over there.”
This first-of-its-kind study highlights the degree to which the cytoplasm is “beautifully organized” rather than being just a big jumble of stuff, she says.
Not only do the findings shed new light on fundamental cellular biology, but the knowledge also holds promise for increasing or altering the production of proteins in mRNA vaccines and therapies, the researchers note.
The study was led by former lab member Ellen Horste, Ph.D., whom Mayr tapped for the daunting but exciting project when she joined the lab several years ago. Dr. Horste received her doctorate from the Gerstner Sloan Kettering Graduate School in June and now works for a gene therapy company.
“When we started, we had a hard time getting funding for this project,” Dr. Mayr says. “Everyone thought isolating the individual components would be totally impossible. This was really Ellen’s project from her first day in the lab to her last day. It was quite challenging, and I couldn’t be more proud of her.”
Adapting an approach commonly used by immunologists, the team was able to color-code individual particles within cells using antibodies and then sort them by color. They used RNA sequencing to identify which RNAs were associated with which particles.
“And it was really striking to see that in each of these intracellular neighborhoods, very different types of mRNAs were being translated,” Dr. Mayr says.
Welcome to the cellular neighborhood
Most of the well-known components inside a cell have a defined shape and come wrapped in an exterior membrane: the nucleus, mitochondria, lysosomes, the Golgi apparatus.
Two of the key components at the heart of the Mayr team’s study don’t have membranes—which is what has made them so hard to find in the first place, and a challenge to isolate and study in the lab.
A quick biology review: Cells build proteins using instructions encoded in DNA. Those DNA sequences are transcribed into mRNA inside the cell nucleus. These messenger RNA then move out into the cytoplasm where they are translated into a useful protein.
The new study demonstrated that where in the cytoplasm this translation step happens isn’t random, and that there’s an underlying logic or “code” that directs mRNAs to specific neighborhoods within the cell.
“The whole cytoplasm is nicely compartmentalized,” Dr. Mayr says. “We were able to demonstrate there is a code at work that’s based on the mRNA’s biophysical features—their size and shape—and the particular RNA-binding proteins they partner with. This code directs the mRNAs to different locations for translation.”
Investigating translation in three locations inside the cell
Through a painstaking series of experiments, the research team was able to show that mRNAs of different lengths and shapes tend to gravitate to specific neighborhoods. And that if you intervene to redirect them to a different location, it can have a profound impact on the amount of protein that gets produced and on the protein’s function.
The researchers looked at mRNAs that are located on the surface of the endoplasmic reticulum (an organelle involved in protein synthesis and other cellular functions). It’s well established that proteins associated with cellular membranes and those that get secreted by the cell for use elsewhere are translated there.
The research revealed that nearly 15% of mRNAs that encode non-membrane proteins are also translated at the ER—and they encode large and highly expressed proteins.
Meanwhile, the mRNAs that get translated in the cytosol (the liquid part of the cytoplasm) tend to be very small proteins.
And mRNAs that locate to TIS granules tend to be transcription factors (proteins that regulate the transcription of genes). TIS granules are a membrane-less cellular component Mayr’s lab discovered in 2018. They form a network of interconnected proteins and mRNAs, and are closely allied with the endoplasmic reticulum, forming a distinct space where mRNA and proteins can collect and interact.
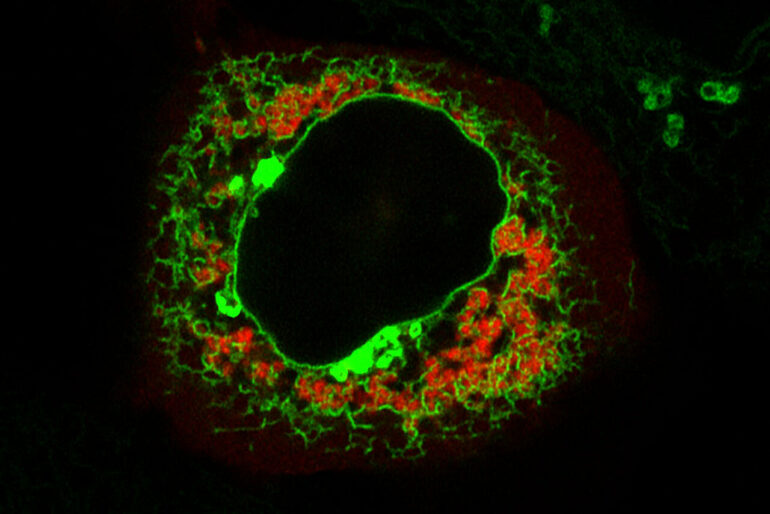
A fluorescent microscopy image of a cell, with TIS granules shown in red and the endoplasmic reticulum is shown in green. The central black area is the cell’s nucleus.
Cracking the code
Cracking the code for how mRNA localizes to different locations revealed some surprising findings.
After discovering the TIS granule network five years ago, the lab turned its attention to understanding which of the many thousands of mRNAs in a cell localize there and whether they have shared characteristics.
The team homed in on one part of the mRNA that doesn’t usually get much attention—the tail. It’s separate from the middle part of the mRNA, which contains the instructions for building the protein. Scientists call the tail the three prime untranslated region (3′ UTR), and it turns out to be critical for the localization process.
“The tail usually contains a longer sequence than the part of the RNA that’s actually used to make the protein,” Dr. Mayr says. “But for a long time, people didn’t pay that much attention to the tail regions since you can still make the protein without them.” (They’re also important in other ways, as Dr. Mayr outlined in a 2019 review article.)
It turns out that the tail is essential for partnering with RNA-binding proteins so that, together, the mRNA goes to the correct translation region within the cell. (RNA-binding proteins are a type of protein that attaches to RNA molecules and can modulate various aspects of their activity.)
At first, the team thought it was primarily these RNA-binding proteins that directed the action—guiding the mRNAs to neighborhood one, neighborhood two, and so forth, Dr. Mayr says.
“But the really surprising finding was that the RNA-binding proteins actually play a secondary role rather than a primary role in the process,” she says.
The default sorting of mRNA to a location, the researchers found, is based on the overall size and shape of the mRNAs. But being in partnership with a binding protein can override this default and redirect them.
“Our data show that if you translate an mRNA in the TIS granules, the resulting protein will perform one function, and if you translate it outside of the TIS granules, it will perform a different function,” she says. “And this is how, in higher organisms like us, one protein can have more than one function.”
Toward future applications
One specific protein the team examined during the study is MYC. The MYC gene is one of the more famous oncogenes, and mutations in MYC underlie the development of many cancers.
“We observed that several MYC protein complexes were only formed when MYC mRNA was translated in the granules and not when it was translated in the cytosol,” Dr. Mayr says. “Our results show there’s an important biological relevance to these neighborhoods, even when only about 20% of mRNAs get translated in the TIS granules.”
Together, these insights suggest that mRNA could be targeted to achieve different functions, as well as to vary the amount of a protein that gets produced, she adds.
“So, we hope that in the future we can make smarter medicines by making more or less of a particular factor, and also by manipulating its function,” Dr. Mayr says. “This probably won’t happen in the next five years, but it’s something we are paving the way to do.”
More information:
Ellen L. Horste et al, Subcytoplasmic location of translation controls protein output, Molecular Cell (2023). DOI: 10.1016/j.molcel.2023.11.025
Provided by
Memorial Sloan Kettering Cancer Center
Citation:
Location, location, location: Research reveals the hidden power of intracellular neighborhoods (2023, December 21)