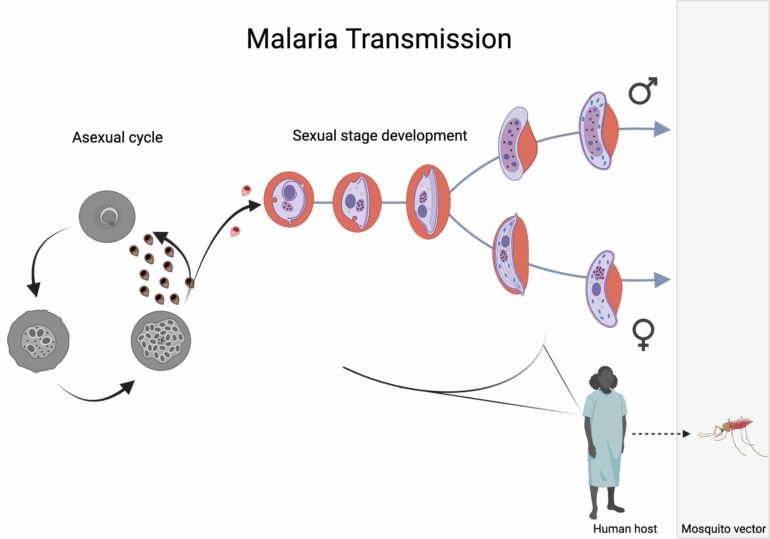
Malaria is caused by a eukaryotic microbe of the Plasmodium genus, and is responsible for more deaths than all other parasitic diseases combined. In order to transmit from the human host to the mosquito vector, the parasite has to differentiate into its sexual stage, referred to as the gametocyte stage.
Unlike primary sex determination in mammals, which occurs at the chromosome level, it is not known what causes this unicellular parasite to form males and females. New research at Stockholm University has implemented high-resolution genomic tools to map the global repertoire of genes of gametocyte development towards the male or the female sexual fates.
The study, published in Nature Communications, uncovers the genes that are expressed in Plasmodium falciparum, the deadliest among the malaria parasites, from the very onset of sexual stage development until they reach maturity. At this point, the male and female gametocytes are ready to be taken up by the female Anopheles mosquito in order to initiate the unrelenting transmission cycle.
“We have combined state-of-the-art single-cell genomics with a novel computational approach to define the expression of several important genetic regulators along the developmental trajectory of male and female gametocytes,” says Johan Ankarklev, Associate Professor at the department of Molecular Biosciences, the Wenner Gren Institute, and senior author of the study.
The research from Stockholm University, performed in collaboration with Dr. Johan Henriksson at MIMS—Umeå University and the Microbial Single Cell Genomics facility at SciLifeLab, is important for improving our understanding of the genetics underlying malaria transmission.
A widely conserved family of transcription factors called the ApiAP2, has emerged as key regulators of gene expression during Plasmodium lifecycle-stage differentiation and development.
“Our high-resolution data enabled us to computationally link the expression of several of these ApiAP2 genes with either the male or the female lineage, implicating their involvement in sexual cell fate determination. Importantly, we also established a large set of novel candidate ‘driver’ genes of the male and the female cell fates, which we are currently further exploring in the lab using CRISPR technology,” Ankarklev continues.
The study contributes important findings to the malaria community but also to the greater scientific community:
From a clinical perspective, treatment strategies have historically targeted the highly symptomatic, asexual blood stage of infection, with variable degrees of success. Importantly, current treatment strategies do not inhibit malaria transmission. This study provides new and important genetic markers for the future development of transmission blocking therapies, which is the only way to inhibit the spread of malaria.
From an evolutionary perspective, considering that Plasmodium is an ancient microbial eukaryote that produces males and females, the new data and analyses contribute novel information and clues regarding the evolution of sex in eukaryotes.
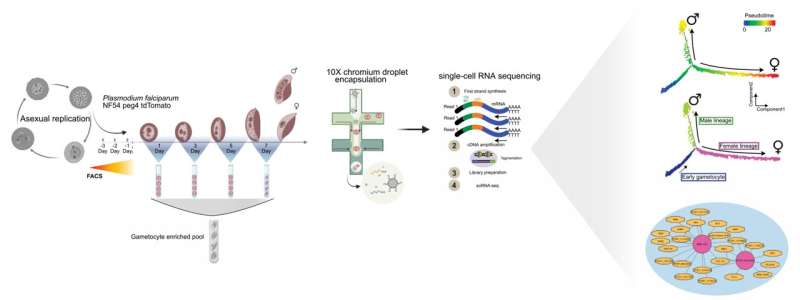
Schematic outline illustrating the experimental approaches used to characterize transcriptional changes during P. falciparum male and female gametocyte development, using the P. falciparum gametocyte-producing NF54 peg4-tdTomato transgenic cell line. The schematic highlights FACS-based parasite population enrichment followed by 10X 3′ single-cell RNA-seq (10X Genomics) and highlights two of the computational analyses in the study. © Stockholm University/Nat Comm graphical abstract/CC BY 4.0
There is currently little knowledge about malaria’s sexual reproduction
Most eukaryotes undergo sexual reproduction to ensure diversity and fitness selection. In animals, sex determination most commonly involves males and females. However, among the vast diversity of organisms that make up the eukaryotic microbes, the systems by which sex is defined are highly diverse and often cryptic.
The malaria parasite, Plasmodium spp., belongs to the Apicomplexan phylum, a group of obligate invasive, unicellular parasites, which form male and female gametes. The French scientist, Alphonse Laveran, first described the crescent-shaped malaria gametocyte in 1880. Two decades later, the British physician, Robert Ross, made the discovery that malaria was transmitted by mosquitoes.
Despite these important discoveries, it is only in recent years that significant progress has been made in improving our understanding of the biology of the malaria transmission stages, thanks to new and groundbreaking biotechnology.
How new genomic tools enable progress in malaria research
Single-cell transcriptome profiling allows a snap-shot of a large array of genes expressed in one cell, in this case one malaria parasite, at a given point of development. When adding thousands of single cell transcriptomes to the analysis, it becomes a powerful tool for determining genetic pathways and developmental bifurcations, which is essential for lineage tracing.
“By combining Pseudotime and RNA Velocity, two recently developed computational tools, we aligned the several thousands of cells along a branched pseudo-time axis. Second, we used RNA velocity estimates to define the splicing kinetics among transcripts across the developmental axes. This then allowed us to predict a large panel of putative ‘driver genes’ for the male and the female sexual fates, and interestingly, a large number of these genes have previously not been annotated,” says Mubasher Mohammed, a former Ph.D. student at the Ankarklev Lab and lead author of the study.
Mohammed grew up in Sudan where he experienced the devastating effects of malaria first hand.
“It is a fun time to be a scientist, where new technologies enable us to make giant leaps in our understanding of different types of diseases that plague humankind,” says Mohammed.
The malaria transmission stage marks a dramatic decrease in the parasite population numbers making it an attractive target for antimalarial control efforts.
“When such a bottleneck occurs in the population, it becomes more vulnerable to drugs and environmental factors. By delineating the molecular mechanisms of gametocyte development, we can target these pathways to develop effective transmission-blocking strategies, vital for malaria eradication efforts,” says Alexis Dziedziech, a previous postdoc at the AnkarklevLab and co-author on the study.
More information:
Single-cell transcriptomics reveal transcriptional programs underlying male and female cell fate during Plasmodium falciparum gametocytogenesis, Nature Communications (2024). DOI: 10.1038/s41467-024-51201-3. www.nature.com/articles/s41467-024-51201-3
Provided by
Stockholm University
Citation:
Mapping the sex life of malaria parasites at single cell resolution reveals genetics underlying transmission (2024, August 26)