As molecular biologists at Boston University and as husband and wife, Ruslan Afasizhev and Inna Afasizheva have worked together for decades. Together, they have published dozens of papers on the mechanics of mitochondrial DNA and RNA in a single-celled, disease-causing parasite called Trypanosoma brucei. Now, years of breakthroughs have led to their latest paper published in Science, which provides a detailed look at a mystifying process called RNA editing and could potentially help treat a deadly disease.
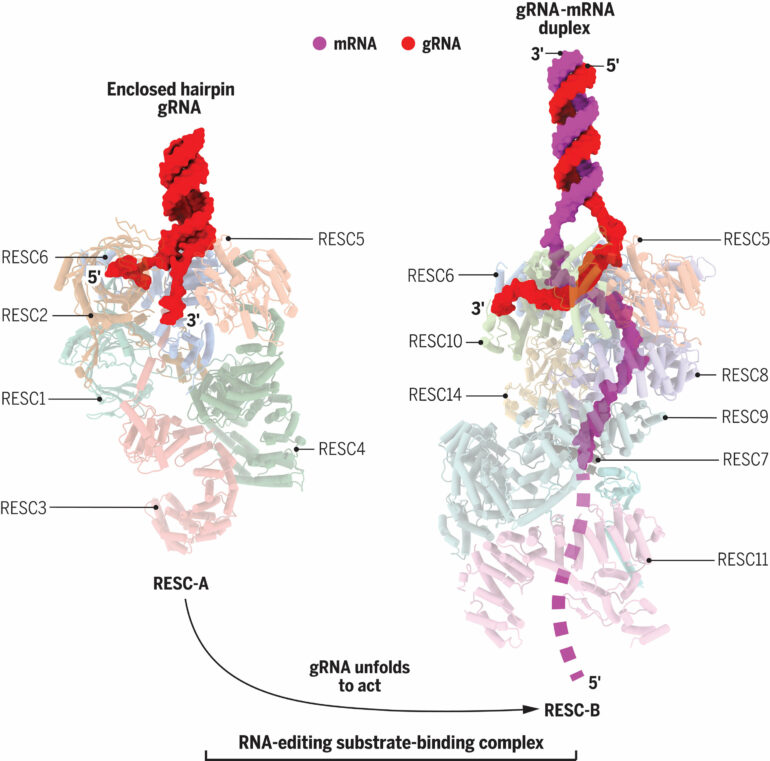
In their newest paper, Afasizhev and Afasizheva—along with collaborators at UCLA, University of California, Irvine (UCI), and ShanghaiTech University—are the first to determine the architecture of the molecular machines that harbor gRNA strands and allow those strands to engage mRNA.
Identifying these cellular mechanisms could give scientists essential information for treating African sleeping sickness, the disease caused by Trypanosoma. Spread by tsetse flies that harbor the parasite, African sleeping sickness is usually fatal, and many of the available treatments have safety concerns, making molecular studies particularly important for drug development.
“Now we can start more broader research,” says Afasizheva, a Boston University Henry M. Goldman School of Dental Medicine associate professor of molecular and cell biology. “Since now we know exactly how proteins interact with RNA.”
“If we find a way to inhibit the editing process, we can kill the parasite without harming human cells,” says Afasizhev, who is also a professor of biochemistry at the Boston University Chobanian & Avedisian School of Medicine and corresponding author on the paper.

The couple share a fascination for small RNA biology—the study of small RNA strands that serve specific functions in a cell. © Cydney Scott
RNA research has evolved and advanced tremendously—and so has the technology to study the inside of cells. Today, the couple’s team uses cryo-electron microscopy and molecular approaches to provide a detailed understanding of RNA editing.
Using this technology, their latest study has found that a protein complex called the editosome is responsible for orchestrating changes guided by gRNA, which happen as a cascade of insertions and deletions of uridine, a chemical component of RNA. In Trypanosoma, RNA editing serves an important purpose: fixing a broken gene. Mutations in DNA are very common in the parasite, so even though the genetic code is unreadable, the edited mRNA becomes a functional part of the cell.
RNA editing regulates many cellular processes in nearly all organisms that have cells with a nucleus and mitochondria. But, Afasizhev says, the RNA editing mechanisms in different organisms have nothing in common, meaning those mechanisms evolved for different purposes specific to different species. This is what makes the RNA editing mechanisms of Trypanosomes an attractive therapeutic target for stopping the parasite from causing disease, since it won’t interfere with human cells. Now that they know the protein structures unique to RNA editing in Trypanosomes, the next phase of their research is identifying the enzymes that ignite the reactions in the cell.
“The next question is how these reactions happen, how these enzymes come to the substrate, and how they create the magnificent work to change the RNA sequence,” Afasizheva says.
She and Afasizhev hope to bring in more students to their lab who can embrace the technological advances in their field, and continue to solve this complicated puzzle, just as they have.
More information:
Shiheng Liu et al, Structural basis of gRNA stabilization and mRNA recognition in trypanosomal RNA editing, Science (2023). DOI: 10.1126/science.adg4725
Citation:
Molecular biologists identify framework for understanding RNA editing in a disease-causing parasite (2023, July 19)