Bacteria of the genus Shigella, closely related to the well-known Escherichia coli, are the second most common cause of fatal bacterial diarrheal diseases, with over 200,000 victims worldwide every year. There are repeated outbreaks of strains that are resistant to common antibiotics.
In order to better control Shigella and other pathogenic bacteria, researchers around the world are looking for new therapeutic targets. Researchers from the Max Planck Institute for Terrestrial Microbiology in Marburg have now provided insights into bacterial gene regulation, which at the same time contribute to a better understanding of the infectious capacity.
A team led by Martin Thanbichler, Max Planck Fellow at the Max Planck Institute for Terrestrial Microbiology and Professor of Microbiology at Philipps-Universität Marburg, recently identified and elucidated a new type of molecular switch that controls the distribution of genetic material during cell division.
It now appears that the same mechanism is also important for the regulation of bacterial genes. The researchers studied the infection process of the bacterial cause of dysentery, Shigella flexneri. They not only clarified a critical new aspect of the underlying regulatory process but also provided important information on how the bacterium uses this mechanism to control its ability to infect. Their findings could open up new avenues for combating this and related bacterial pathogens.
The molecular switch that the team discovered a few years ago has an unusual property: its activation and deactivation are based on the ribonucleotide CTP (i.e., the RNA building block cytidine triphosphate; the chemically related adenosine triphosphate, ATP, is also known as the universal energy carrier of biological processes).
Since the ability to hydrolyze CTP is likely present in many bacterial proteins, the researchers suggest that it represents a widespread, previously undiscovered control principle. Such a principle could also affect bacterial processes relevant to humans. Indeed, the researchers discovered that the regulation of virulence genes in Shigella is mediated by a CTP-dependent switch that is central to the infection process.
Shigella and related bacteria owe their ability to infect and their resistance to antibiotics to a special feature: plasmids, circular DNA molecules that are inherited or passed on independently of the actual genome.
As early as 20 years ago, a protein called VirB was discovered that, as a transcription factor, controls the transcription of several gene clusters on the Shigella virulence plasmid and thus the ability of Shigella to infect human intestinal cells. Despite many years of research, the mechanism by which VirB controls gene expression remained unclear.
A ‘remote control’ of Shigella’s ability to infect
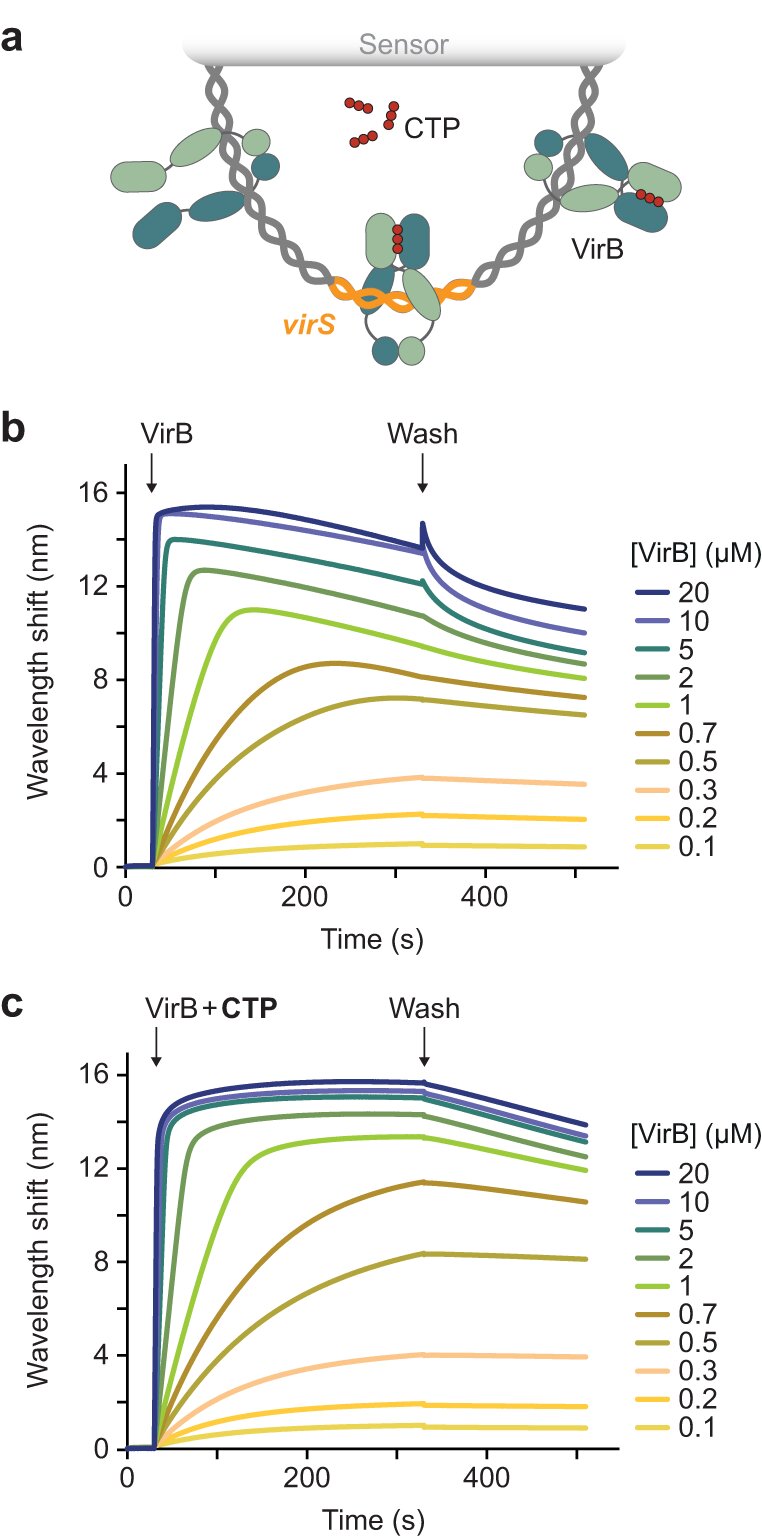
Remarkably, VirB is not a classical transcription factor, but is related to the same class of proteins as the CTP-dependent switches that help to smoothly distribute the genetic information of bacteria to daughter cells during cell division. “The site where VirB binds to DNA is surprisingly far away from its target genes, and it was unclear how it could be active over such large distances,” explains Sara Jakob, first author of the study.
“Bioinformatic structure predictions indicated that VirB might be able to bind CTP. Our studies then showed that it indeed uses a CTP-dependent switch mechanism to control the expression of virulence genes,” adds Martin Thanbichler.
Using a broad methodological approach, the researchers were able to elucidate the mechanism: The protein interacts with its binding site on the DNA and wraps itself in a ring around the DNA molecule, with CTP holding the VirB ring in a closed state like double-sided tape. In this form, VirB then slides along the side of the DNA, making the binding site accessible again and allowing more VirB molecules to be loaded.
These then modify the structure of the DNA so that the target genes can be read. This CTP-dependent loading and sliding mechanism enables VirB to act as a molecular switch to remotely control gene expression during bacterial pathogenesis.
CTP dependence is a potential target for novel therapies
“Mutations that prevent the binding of CTP inhibit the loading of VirB onto DNA in vitro and suppress the formation of VirB-DNA complexes as well as the expression of virulence genes in Shigella cells,” explains Sara Jakob.
Since this type of switch does not occur in humans, VirB could be a target for novel therapeutics that specifically suppress Shigella virulence and thus enable a better treatment of shigellosis, says Martin Thanbichler. “Our work provides the first evidence for a CTP-dependent switch involved in gene regulation. It shows that this newly discovered regulatory principle is of far-reaching importance for the control of biological processes in bacteria.”
The study is published in the journal Nature Communications.
More information:
Sara Jakob et al, The virulence regulator VirB from Shigella flexneri uses a CTP-dependent switch mechanism to activate gene expression, Nature Communications (2024). DOI: 10.1038/s41467-023-44509-z
Provided by
Max Planck Society
Citation:
Molecular switch found to play central role in bacterial dysentery (2024, January 19)