A team from Children’s Medical Research Institute has discovered a new way to impair cancer cell growth, which could lead to the development of a new class of cancer therapeutics with minimal side-effects on normal cells.
Professor Hilda Pickett, who leads CMRI’s Telomere Length Regulation Unit, said this was an exciting development for their team.
“This project builds on over a decade of work by my team and our collaborators to develop antiproliferative agents that target telomeres. The results are promising and have future potential to improve long term health outcomes for all types of cancer,” says Pickett.
Our DNA is protected by telomeres, found at the ends of our chromosomes, which form a looped structure guarded by an array of proteins, with one of the most crucial being TRF2. When this protective telomere structure is disrupted, there are catastrophic effects on cells, causing them to die.
Cancer cells exist on the edge of catastrophe. The unlimited proliferative capacity of cancer cells is entirely dependent upon telomere maintenance, making factors that control telomeres attractive chemotherapeutic targets. Anything that tips them over the edge can selectively kill cancer cells without harming the normal cells in our body. Unlike radiation and most chemotherapies, the side effects of telomere-focused cancer drugs are predicted to be much lower, improving patient survival and long-term outcomes.
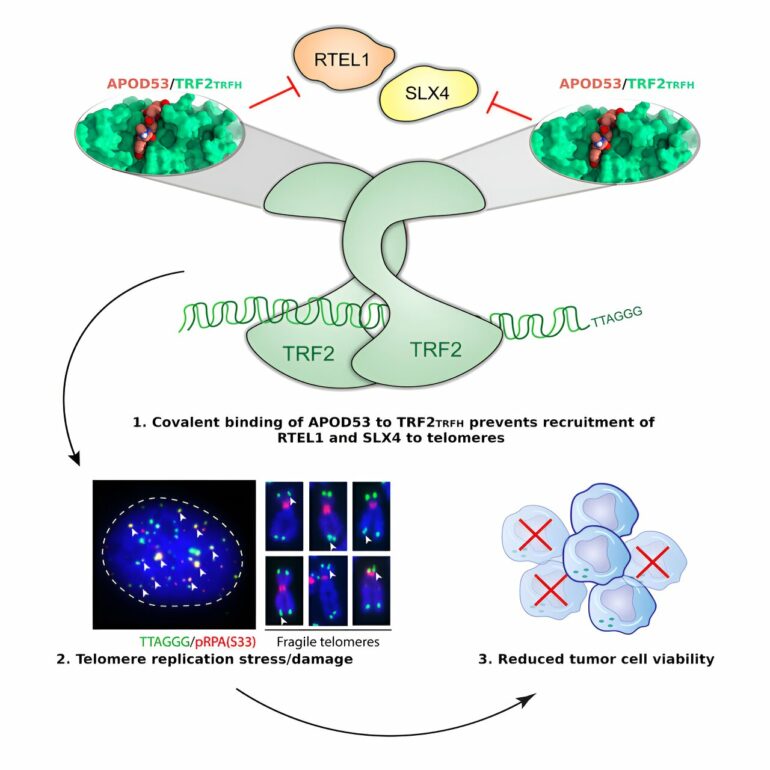
CMRI’s Dr. Alex Sobinoff, a member Professor Pickett’s team, is lead author on a paper published in Cell Chemical Biology, which describes the first compound to strongly bind a key region of TRF2. This compound, called APOD53, impairs cell growth in cancer cells (using both known telomere maintenance mechanisms—ALT and telomerase), while sparing non-cancerous cells.
“These compounds represent fantastic new tools for fundamental research and prove that we can develop cancer therapeutics that specifically target TRF2,” says Sobinoff.
They have also shown the effectiveness of their new compound can be increased in combination with other chemicals affecting other parts of the protective structure on telomeres. What’s more, their compound, a peptide, is permeable, meaning it can easily enter cells and is thus a good candidate for future anti-cancer drug development.
“The success of these early-stage compounds can be attributed to the intelligent drug design approach we employed, combined with our in-depth understanding of telomere biology,” say the researchers.
More information:
Alexander P. Sobinoff et al, Irreversible inhibition of TRF2TRFH recruiting functions by a covalent cyclic peptide induces telomeric replication stress in cancer cells, Cell Chemical Biology (2023). DOI: 10.1016/j.chembiol.2023.11.008
Provided by
Children’s Medical Research Institute (CMRI)
Citation:
New compound could lead to therapeutics that selectively kill cancer cells (2023, December 8)